23rd October 2022, Dr Chee L Khoo

The UKPDS 33 demonstrated that intensive glucose control had significant microvascular benefits over a 10-year period in patients with newly (< 6 years) diagnosed type 2 diabetes (1). Overall, there was a trend towards macrovascular benefits during that period but did not reach statistical significance. The participants were followed up for another 10 years after the study was published in 1997. The microvascular benefits were still statistically significant after another 10 years despite the intensive group’s glycaemic control deteriorating in the later years to approach the control group’s glycaemic control. This is often labelled as the “legacy effect”. Further, after the subsequent 10 years, macrovascular benefits became statistically significant. It’s been 44 years since the participants started in the study back in 1977. A preliminary report on the 44-year outcomes was presented recently at the EASD in Stockholm. It has not been published.
We are so used to quoting from the UKPDS study for so long now that it might be prudent to revisit the original UKPDS study details before we dive into the 44-year follow up report. I always thought that there were two groups – the intensive glucose intervention group vs the conventional care group. In fact, they had 3 groups – the intensive glucose intervention group utilising sulphonyurea and/or insulin, another intensive glucose intervention group utilising metformin and the conventional care group. (see Figure 1). The final results were always reported as the intensive group(s) combining the SU/insulin data with the metformin data. It is insightful to tease out the differences between the two intensive groups.
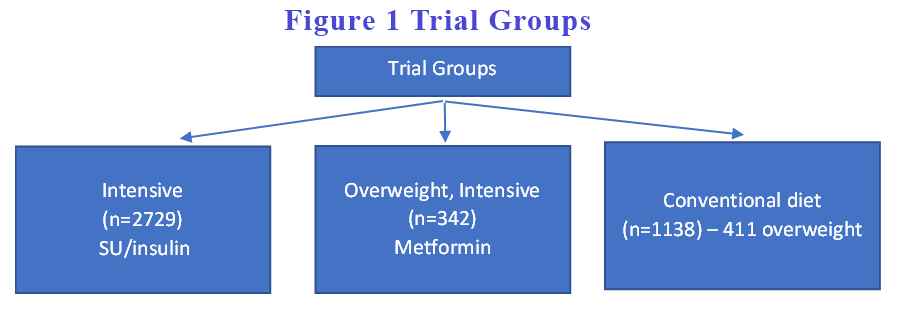
Interestingly, the often quoted “intensive blood glucose control substantially reduce the risk of microvascular complications but not macrovascular disease” primarily relates to intensive control with SU/insulin. When we look at intensive glucose control with metformin group, the microvascular complications were not significant but the macrovascular benefits were. Because the number of participants in the metformin group were small, the effect was diluted in the overall composite data analysis. Further, the reduction in all-cause mortality was statistically significant in the metformin but not in the SU/insulin group.
After the intervention was completed and the intervention ended in 1997, the participants were monitored for another 10 years. The follow up study was published in 2007. While the glucose control was better in the intensive glucose control group compared with the conventional group in the first 10 years of the study, the difference in glucose control between groups were lost as the conventional group improved and the intensive group deteriorated over the years. All diabetes endpoints continue to be better in the intensive group despite a deterioration in their glucose control in subsequent years. That is often referred to as the “legacy effect”.
The post-trial monitoring did not end in 2007. Participants continued to be monitored for another 14 years (see Figure 2). Data were collected from the NHS Digital which primarily consist of hospital admissions, outpatient appointments, attendance at accident and emergency departments and deaths. It did not include any primary care data. Only data from England were presented as they await data from Scotland and Northern Ireland.
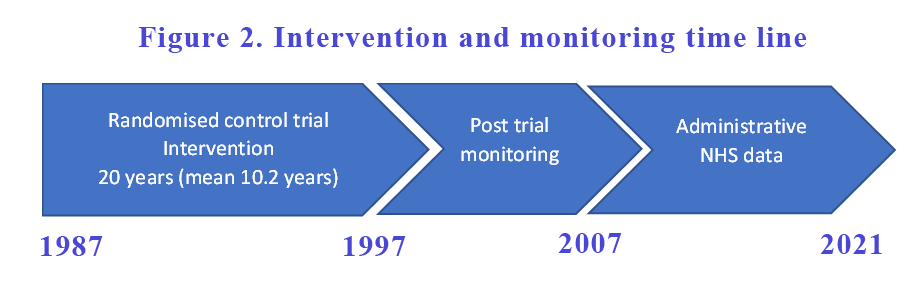
They compared groups that were previously allocated – SU/Insulin vs conventional and Metformin vs conventional groups. They compared 4 aggregate complications of diabetes (any diabetes-related endpoints, all-cause mortality, myocardial infarction and microvascular complications), health economics and all-cause dementia between the groups.
After 44 years

Despite the passage of time and the similarities in their glycaemic control after the first 10 years, when they looked at any diabetes-related endpoint, there was still a statistically significant 10 % reduction in the SU/Insulin group compared to conventional group. When they looked at AMI specifically, they had a statistically significant 15% reduction in the SU/insulin group compared with the conventional group. Similarly, there was a statistically significant 26% reduction in microvascular complications between the groups. All cause mortality was also better by 11% in the SU/Insulin group compared to conventional group. The difference between groups were pretty much similar to those back in 1997 and 2007 suggesting the benefits were maintained with the exception of all cause mortality which was not significant in the original 1997 report.
Remarkably, the benefits of metformin vs conventional care were very similar to the benefits of SU/Insulin over conventional care except for microvascular complications where metformin did not have any significant benefits over the conventional group. Howevere, the all-cause mortality benefit of metformin which was present even after 10 years were maintained into the 44th year.
Legacy effect according to HbA1c
Compared with a 50-year-old patient whose HbA1c was left at 8% for the first 20 years, the same patient whose HbA1c was 8% for the first 10 years but was reduced to 7% for the subsequent 10 years, there was a 6.6% relative risk reduction (RRR) in death. If that patient had the HbA1c reduced to 7% right at the start and kept < 7.0% for the next 20 years, there was a 18.6% reduction in RRR! (see Figure 4).

Life expectancy gains including QALYs gained
The term “quality-adjusted life year” or “QALY” is a measure of health outcomes pertaining to disease burden and is used to assess the value of medical interventions. As health can be defined as the length of life and the quality of life, the QALY combines the two factors into a single figure.
In other words, quality-adjusted life year measures how many additional months or years of life of a reasonable quality a patient or person may gain due to treatment.
When they compared the life expectancy of the SU/Insulin group with the conventional group, the SU/Insulin group gained 1.1 (0.3-2.0) years. The SU/Insulin group also gained 0.9 (0.2 – 1.5) QALYs compared with the conventional group. Participants on metformin gained 2.7 (1.0 – 4.4) years of life and 2.0 (0.7 – 13.2) QALYs compared to the conventional group.
Dementia
Patients with diabetes have a 60% higher risk of all forms of dementia (3). Most glucose lowering agents except insulin reduce the risks although the results are conflicting because most of the studies are too small or too short. There are only a few ongoing trials looking at effect of glucose lowering agents on dementia.
In the 44-year follow up of the UKPDS, the effect of 10 years of treatment with SU/Insulin range from 4% increase to a 29% reduction when compared with conventional treatment. The effect of 10 years of treatment with metformin range from an 18% increase to a 49% reduction when compared with conventional treatment. The effects were not statistically significant. The authors did acknowledge that they did not have primary care data and dementia was not always recorded.
In summary, the legacy effect of early glycaemic control first identified in the UKPDS 30-year analyses remain essentially unchanged up to 44 years of follow-up. Early intensive treatment with SU/Insulin led to a 11% reduction in deaths and 26% reduction in microvascular complications. Early intensive treatment with metformin led to a 25% reduction in deaths and 31% reduction in myocardial infarction. These landmark findings emphasise the importance of diagnosing diabetes early and treating intensively from day 1.
It is thought that suboptimal glycaemic control induces irreversible pathophysiological changes which permanently increasing the risk of diabetic related complications and premature death. Metformin, in addition to reducing hyperglycaemia, has additional legacy effect suggesting that metformin may have other protective mechanisms.
References:
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837-53. [Erratum, Lancet 1999;354:602
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008 Oct 9;359(15):1577-89. doi: 10.1056/NEJMoa0806470. Epub 2008 Sep 10. PMID: 18784090.
- Saion Chatterjee, Sanne A.E. Peters, Mark Woodward, Silvia Mejia Arango, G. David Batty, Nigel Beckett, Alexa Beiser, Amy R. Borenstein, Paul K. Crane, Mary Haan, Linda B. Hassing, Kathleen M. Hayden, Yutaka Kiyohara, Eric B. Larson, Chung-Yi Li, Toshiharu Ninomiya, Tomoyuki Ohara, Ruth Peters, Tom C. Russ, Sudha Seshadri, Bjørn H. Strand, Rod Walker, Weili Xu, Rachel R. Huxley; Type 2 Diabetes as a Risk Factor for Dementia in Women Compared With Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care 1 February 2016; 39 (2): 300–307.
