
27th November 2020, Dr Chee L Khoo
Every so often you come across reports of some “ultra-rare” disease that has a treatment available now and you probably thought that it really doesn’t concern you or your patients. In a recent small study reported in The Lancet, Karine Clément, Erica van den Akker et. al. presented the results of setmelanotide in participants with proopio-melanocortin (POMC) gene mutation which is associated with severe childhood obesity. The number of individuals in the USA estimated to have genetic mutations in the melanocortin pathway is estimated to be 12 800 (1). While our knowledge of the genetic aspects of obesity is far from complete, these mutations deepen our understanding on the roles various hormones and neuropeptides play in regulating appetite and their contribution to severe obesity. It has also allowed the pathway as a potential therapeutic target in the management of severe obesity.
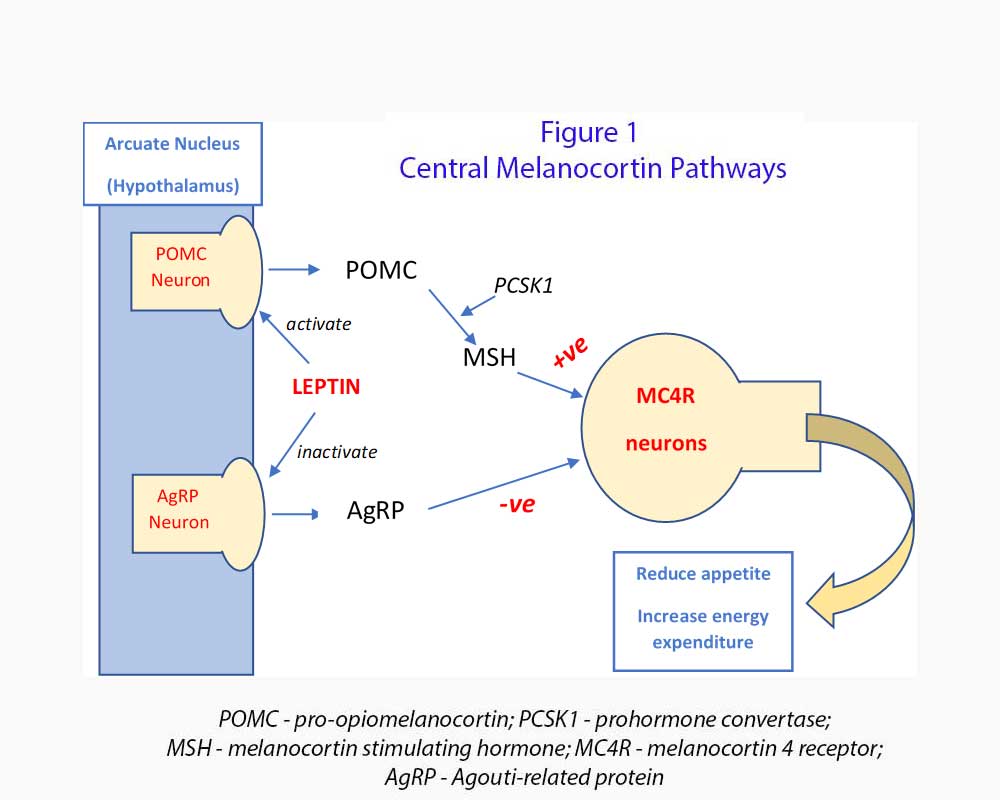
The Melanocortin system (see Figure 1)
Before we look at what setmelanotide can do for these patients, we need to review the melanocortin pathway. The role the pathway play in metabolism was not worked out when most of us graduated from medical school. Let’s look at the top of the tree in the hypothalamus.
The hypothalamus plays a critical role in controlling food intake and energy expenditure. In particular, the arcuate nucleus, located in the mediobasal hypothalamus has an aggregate of projecting and neuro-endocrine neurons which regulate feeding, energy expenditure and metabolism. They can execute their function either directly onto other parts of the brain or body or by working through the pituitary gland
The central melanocortin system consists of neurons located within the arcuate nucleus that release orexigenic (hunger generating) neuropeptides agouti-related protein (AgRP) and neuropeptide Y (NpY). AgRP is an endogenous melanocortin inverse agonist which inhibits melanocortin receptors especially melanocortin 4 receptor (MC4R).
Also located within the arcuate nucleus are anorexic neurons which express POMC. POMC is a precursor polypeptide which is cleaved into melanocyte stimulating hormone (α-MSH and β-MSH), adrenal corticotropic hormone (ACTH), and β-endorphin by prohormone convertase 1 (PCSK1). MSH activate MC4R in the second order neurons and induce satiety, increase energy utilisation and promote weight loss. PCSK1 (together with PCSK2) also helps to process proinsulin and glucagon in the pancreatic islet cells.
POMC neurons are activated by leptin (via leptin receptor, LEPR) and insulin while AgRP neurons are inhibited by leptin and insulin. Severe obesity which starts from 1 year old occurs when there is POMC deficiency caused by POMC or PCSK1 genetic variants which leads to deficiency of MSH. MC4R is not activated and these patients have hyperphagia and early onset severe obesity.
Variants in POMC can also result in adrenocorticotropic hormone deficiency, hypothyroidism, hypogonadism and hypopigmentation due to loss of POMC-derived melanocortin peptides. Variants in PCSK1 can result in increased proinsulin and postprandial hypoglycaemia, hypogonadism, hypocortisolism, and malabsorption due to impaired prohormone processing. Variants in LEPR can also result in hypogonadism, hypothyroidism, growth hormone deficiency, high infection risks and sepsis related mortality, possibly from impaired immune function (2-7).
Setmelanotide is an octapeptide that binds to and activates multiple melanocortin receptors: MC4R, MC3R and MC1R selectively over MC5R and MC2R. Multiple MC4R agonists have been studied as potential anti-obesity medications (8). Some of these activate the sympathetic nervous system with blood pressure elevation and increased heart rate making them unacceptable in clinical care although setmelanotide has not shown this characteristic (9).
Between Feb 14, 2017, and Sept 7, 2018, ten participants were enrolled in the POMC trial and 11 participants were enrolled in the LEPR trial (10). The POMC trial included individuals aged 6 years or older with obesity caused by POMC deficiency, defined as homozygous or compound heterozygous variants in POMC or PCSK1, and a BMI of at least 30 kg/m² (for >18 years) or a bodyweight of more than the 95th percentile for age on growth chart assessment (in those 6-18 years). The LEPR trial included individuals aged >6 years with obesity caused by LEPR deficiency, defined as homozygous or compound heterozygous variants in LEPR, and the same BMI and bodyweight criteria as described for the POMC trial.
Setmelanotide was administered subcutaneously daily and titrated till weight loss was 2-3kg per week for adults and 1-2 kg per week for children. The primary endpoint was the proportion of participants who achieved at least 10% weight loss compared with baseline at approximately 1 year.
Weight loss of at least 10% was observed in 80% of participants in the POMC trial and 45% in the LEPR trial, and the mean percentage change in bodyweight at approximately 1 year was greater in participants in the POMC trial than in the LEPR trial (−25·6% vs −12·5%).
There were no increases in heart rate or blood pressure in this study, but the most frequent side-effect in the POMC trial was injection site reaction and hyperpigmentation. Hyperpigmentation might make the drug undesirable from a patient perspective. In the LEPR trial, the most commonly reported treatment-related adverse events were injection site reaction in all 11 participants, skin disorders in five participants, and nausea in four participants.
Overall, setmelanotide seems a lot more promising for patients with POMC deficiency than in patients with LEPR deficiency. There are ongoing efforts to recruit patients with other genetic obesity syndromes e.g. Smith-Magenis syndrome and Alström syndrome, for treatment with setmelanotide. Having a drug that is effective would then drive clinicians to increase genetic testing for patients with a history of severe early-onset obesity.
Thus, setmelanotide may renewed appreciation for the biological underpinnings of obesity and an increase in genetic screening to identify other subsets of patients that can benefit from the drug. It also further our understanding of the genetics of obesity.
References:
- Ayers KL, Glicksberg BS, Garfield AS, Longerich S, et al. Melanocortin 4 receptor pathway dysfunction in obesity: patient stratification aimed at MC4R agonist treatment. J Clin Endocrinol Metab 2018; 103: 2601–12.
- Clément K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 1998; 392: 398–401.
- Martín MG, Lindberg I, Solorzano-Vargas RS, et al. Congenital proprotein convertase 1/3 deficiency causes malabsorptive diarrhea and other endocrinopathies in a pediatric cohort. Gastroenterology 2013; 145: 138–48.
- Farooqi IS, Wangensteen T, Collins S, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 2007; 356: 237–47.
- Tschöp J, Nogueiras R, Haas-Lockie S, et al. CNS leptin action modulates immune response and survival in sepsis. J Neurosci 2010; 30: 6036–47.
- Huvenne H, Le Beyec J, Pépin D, et al. Seven novel deleterious LEPR mutations found in early-onset obesity: a ΔExon6-8 shared by subjects from Reunion Island, France, suggests a founder effect. J Clin Endocrinol Metab 2015; 100: E757–66.
- Nunziata A, Funcke J-B, Borck G, et al. Functional and phenotypic characteristics of human leptin receptor mutations. J Endocr Soc 2018; 3: 27–41.
- Kleinendorst L, Abawi O, van der Kamp HJ, et al. Leptin receptor deficiency: a systematic literature review and prevalence estimation based on population genetics. Eur J Endocrinol 2020; 182: 47–56.
- Sharma S, Garfield AS, Shah B, et al. Current mechanistic and pharmacodynamic understanding of melanocortin-4 receptor activation. Molecules 2019; 24: 1892.
- Clément K, van den Akker E, Argente J, Bahm A, Chung WK, Connors H, De Waele K, Farooqi IS, Gonneau-Lejeune J, Gordon G, Kohlsdorf K, Poitou C, Puder L, Swain J, Stewart M, Yuan G, Wabitsch M, Kühnen P; Setmelanotide POMC and LEPR Phase 3 Trial Investigators. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020 Dec;8(12):960-970. doi: 10.1016/S2213-8587(20)30364-8. Epub 2020 Oct 30. PMID: 33137293.
