12th May 2024, NIA Diagnostic Imaging
Developmental dysplasia of the hip (DDH) stands out as one of the most prevalent musculoskeletal disorders manifesting in neonates and infants (Charlton et al., 2017). Owing to the dynamic potential for hip remodelling, hip dysplasia at birth may either resolve spontaneously or progress as the child grows (Loh & Wollett, 2021). DDH encompasses a spectrum of hip conditions, including abnormal acetabular development and hip dislocation causing disruption to the typical alignment of the femoral head and acetabulum as a stable ball-and-socket joint (Landes, 2011). The incidence of DDH is estimated to be around 1.5 – 2 per 1000 births (Kang & Koo, 2017). To mitigate adverse long-term effects or complications of DDH, timely detection and management is crucial (Gyurkovitis et al., 2019).
Risk factors for DDH include:
• Family history of DDH
• Breech presentation
• Female
• Oligohydramnios
• Talipes
• Birth weight over 4kg
• Congenital torticollis
• Foot deformities
(Grissom & Harcke, 2017; Landes, 2011)
Clinical indications and patient history
Clinical assessment for DDH relies on the Barlow and Ortolani tests, performed by trained medical professionals (Williams, 2018). The Barlow test involves flexing the patient’s hips and adducting their legs, followed by applying posterior-lateral pressure to assess for hip dislocation (Williams, 2018). The Ortolani test, on the contrary, entails flexing the hips and abducting the legs, applying anterior pressure to detect if a dislocated hip can be reduced back into the acetabulum (Williams, 2018).

Indications for imaging:
• Family history of DDH
• Breech delivery
• Clicky hip
• Limited hip abduction
• Asymmetric thigh/gluteal crease
• Leg length discrepancy
• Walking/gait abnormalities
(American College of Radiology [ACR] 2023; Landes, 2011).
Ultrasound as a primary imaging modality for DDH:
Ultrasound is the primary imaging modality for patients under 6 months of age, as it allows for multiplanar visualization of the cartilaginous components of the femoral head and acetabulum, along with dynamic assessment of hip stability (Grissom & Harcke, 2017). The effectiveness of ultrasound decreases beyond 6 months due to femoral head ossification causing acoustic shadowing, necessitating x-ray imaging for older children (Landes, 2011), however has been shown to be relatively effective up to 12 months (Kang & Koo, 2017).
At NIA Diagnostic Imaging, our highly experienced sonographers use the Harcke method for DDH imaging which involves dynamic evaluation of the hip. This technique includes the scanning of the hip in the coronal and transverse plane at rest and with stress applied, with the aim of assessing both acetabular shape and hip joint laxity (ACR, 2023; Kang & Koo, 2017).
Neonatal hip anatomy and normal sonographic appearances
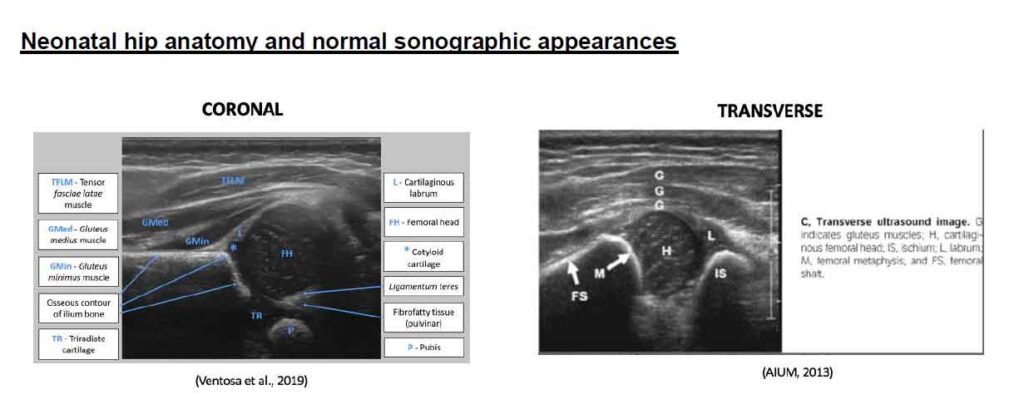
Landmarks for the coronal view of paediatric hips can include the following:
• The Ilium is seen as a hyperechoic straight horizontal line.
• The femoral head is a rounded hypoechoic structure and seen at its largest size. It should normally sit in the acetabulum at rest and with stress application.
• The acetabular margin must have a sharp appearance for normal hips
• The triradiate cartilage is the junction between the ilium, pubis and ischium which must be demonstrated on the image.
• The pubis is at the posterior aspect and its visualization indicates the correct coronal plane has been achieved.
(Landes, 2011).
The main landmarks for the transverse view include the femoral shaft, femoral head and ischium. The image displays a normal hip where the femoral capital epiphysis is located with the U configuration midline between the femoral metaphysis and ischium (Landes, 2011). Application of stress on a normal hip will not disrupt the relationship of the femoral head to the acetabulum (Landes, 2011).
CASE STUDY
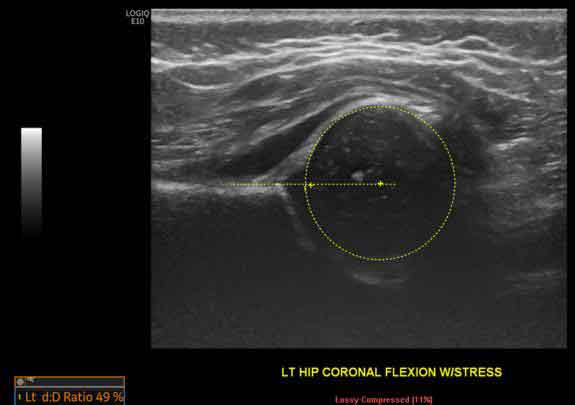
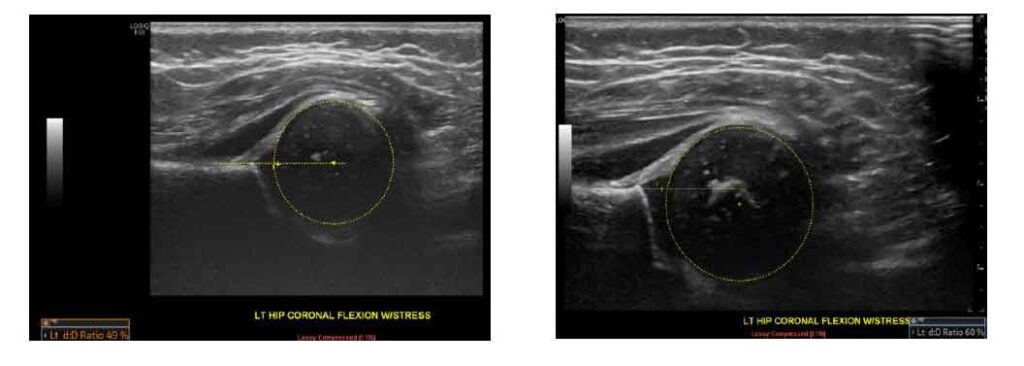
A 6-week-old female neonate presented for a hip US to assess for DDH, due to positive family history. The initial US scan demonstrated a femoral head coverage of 49% as well as acetabular rim rounding which is classified as mild hip dysplasia. Follow-up imaging at 4 months of age demonstrated improvement showing a femoral head coverage of 60% and steeper acetabular rim appearance.
Follow-up and treatment
Ultrasound is used routinely as a follow-up imaging modality to monitor borderline cases of DDH, to assess hip position during treatment or for post treatment progress (Grissom & Harcke, 2017). Treatment options vary according to the severity of hip dysplasia and the age of the patient at diagnosis including; physical therapy (e.g., exercises), harness/brace wearing or surgical procedures to stabilise the hip joint in the correct position (Kang & Koo, 2017). Pavlik harnesses are commonly used for patients under 6 months of age with high successful rates (Kang & Koo, 2017). Closed reduction and casting are used for those aged from 6-18 months, while open reduction is often necessary for children aged over 2 years (Kang & Koo, 2017).
Conclusion
At NIA Diagnostic Imaging our highly experienced staff members perform dynamic US imaging by applying stress maneuvers and conduct measurements of femoral head coverage in their clinical practice. We have highly trained sonographers who specialise in paediatric ultrasound, allowing our Radiologists to make an accurate report.
At NIA Diagnostic Imaging we acknowledge the importance of early diagnosis of DDH so that patients are able to have improved clinical outcomes and aim to reduce the need for invasive treatment methods. As such, we offer same day appointments and accept walk-in patients to ensure early diagnosis and improved quality of life for our patients.
REFERENCES
* American College of Radiology. (2023). ACR–AIUM–SPR–SRU practice parameter for the performance of the ultrasound examination for detection and assessment of developmental dysplasia of the hip. Retrieved 29 March 2024, from https://www.acr.org/-/media/ACR/Files/Practice-Parameters/us-hip-dys.pdf
* American Institute of Ultrasound in Medicine. (2013). AIUM practice guideline for the performance of an ultrasound examination detection and assessment of developmental dysplasia of the hip. Journal of Ultrasound in Medicine, 32(7), 1307-1317. doi:10.7863/ultra.32.7.1307
* Charlton, S. L., Schoo, A., & Walters, L. (2017). Early dynamic ultrasound for neonatal hip instability implications for rural Australia. BMC Pediatrics, 17(82). doi:10.1186/s12887-017-0830-z
* Grissom, L. E., & Harcke, H. T. (2017). The paediatric hip and other musculoskeletal ultrasound applications. In C. M. Rumack & D. Levine (Eds.), Diagnostic ultrasound (5th ed., pp. 1920-1941). Elsevier.
* Gyurkovits, Z., Sohár, G., Baricsa, A., Németh, G., Orvos, H., & Dubs, B. (2019). Early detection of developmental dysplasia of hip by ultrasound. HIP International, 31(3), 424-429. doi:10.1177/1120700019879687
* Håberg, Ø., Bremnes, T., Foss, O. A., Angenete, O., Lian, Ø. B., & Holen, K.J. (2022). Children treated for developmental dysplasia of the hip at birth and with normal acetabular index at 1 year: How many had residual dysplasia at 5 years? Journal of Children’s Orthopaedics, 6(3), 83-190. doi:10.1177/18632521221106376
* Kang, Y. R., & Koo, J. (2017). Ultrasonography of the pediatric hip and spine. Ultrasonography, 36(3), 239-251. doi:10.14366/usg.16051
* Landes, C. J. (2011). Paediatric musculoskeletal imaging. In P. L. Allan, G. M. Baxter, & M. J. Weston (Eds.). Clinical ultrasound (3rd ed., pp. 1497-1513). Elsevier.
* Loh, B., & Wollett, E. (2021). Update on the management of infant and toddler developmental dysplasia of the hip. Australian Journal of General Practice, 50(4). doi:10.31128/AJGP-07-20-5543
* Ventosa, A. R., Alves, P. M. G., Linhares Moreira, A. S., & Patricio, H. (2019). Ultrasound screening for developmental dysplasia of the hip: indications, method and illustrative cases. Retrieved 20 March, 2024, from https://epos.myesr.org/poster/esr/essr2019/P-0168
* Williams, N. (2018). Improving early detection of developmental dysplasia of the hip through general practitioner assessment and surveillance. Australian Journal of General Practice, 47(9). doi:10.31128/AJGP-03-18-4524
