24th February 2025, A/Prof Chee L Khoo
This is one of the most common questions asked at glucagon-like peptide 1 receptor agonist (GLP1-RA) CPD meetings. “What about the risk of thyroid cancer, doc?” As usual, the evidence is rather conflicting. The worry about the thyroid cancer risk with GLP1-RA is actually not without justification. The hypotheses is actually biologically plausible. There have been previous reports of an increased thyroid cancer risk with GLP1-RAs although a recent study published in the journal, Thyroid, provide some reassurance. Is that enough to reassure our patients? We better get to the bottom of this by going back to the beginning.
Concerns about thyroid cancer with GLP1-RA use were first raised in the premarketing phase after studies showed increased rates of thyroid C cell tumours in rodents (1). Glucagon-like peptide 1 receptors are more prominently expressed in papillary thyroid cancer cells than in normal thyroid cells (2,3). In rodents, C-cell hyperplasia is considered a preneoplastic lesion leading to medullary thyroid cancer. Studies in rats and mice showed an increase in the occurrence of benign C-cell adenomas at liraglutide doses that are similar to those seen in humans at the approved doses. There was a statistically significant increase in incidence of malignant C-cell carcinomas among male rats treated with liraglutide at doses eight times those seen in humans receiving the maximum proposed liraglutide dose (4,5).
Remember, these are in pre-clinical studies (i.e. Phase 0 trials) in rats. The relevance of these findings to humans is unknown. Back in 2010, the US Food and Drug Authority (FDA) concluded that increases in the incidence of carcinomas among rodents translated into a low risk for humans, because statistically significant increases occurred only at drug-exposure levels many times those anticipated in humans.
To put the numbers into context, the incidence of medullary thyroid cancer in the United States is approximately 600 cases per year. In Australia, 2,744 women and 1,041 men are estimated to be diagnosed with Thyroid Cancer during 2020 (6). Thyroid cancer is the 9th most common cancer diagnosed in Australia in 2016 and the numbers in 2020 is expected to be the same. Papillary (70%) and follicular (25%) cancers make up 95% of thyroid cancers and medullary make up 4% (2). That translates to 80 cases of medullary cancers in women and 31 cases in men per year in Australia.
All very reassuring but the FDA required additional studies in animals and the establishment of a cancer registry to monitor the annual incidence of medullary thyroid cancer over the next 15 years. In the United States, there is a boxed warning of the possible risk, and those with personal or family histories of medullary thyroid cancer or multiple endocrine neoplasia syndrome type 2 are therefore advised to avoid liraglutide, dulaglutide, exenatide extended release, and semaglutide.
Well, life is as simple as that though. There was an increased number of thyroid cancers in the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) clinical trial evaluating liraglutide versus placebo, but the risk did not reach statistical significance (hazard ratio [HR] 1.66, 95% CI 0.40–6.95), as well as in a meta-analysis of 12 other clinical trials with liraglutide (odds ratio [OR] 1.54, 95% CI 0.40–6.02) (7,8).
In a retrospective cohort study, supplemented with a nested case-control study, using two US administrative databases, Liang et al (2019) found a non-significant trend toward increased risk of thyroid cancer with exenatide (OR 1.46, 95% CI 0.98–2.19) (9).
In a nested case-control analysis performed with use of the French national health care insurance system (SNDS) database, Julien Bezin et al (2023), compared individuals with T2D, but not on GLP1-RA with those on GLP1-RA (10). The use of GLP-1 RA for 1–3 years was associated with increased risk of all thyroid cancer (adjusted hazard ratio [HR] 1.58, 95% CI 1.27–1.95) and medullary thyroid cancer (adjusted HR 1.78, 95% CI 1.04–3.05) (). Interestingly, the risk reduced after >3 years of use. There were no increase in thyroid cancers with DPP4 inhibitors. See Figure 1.
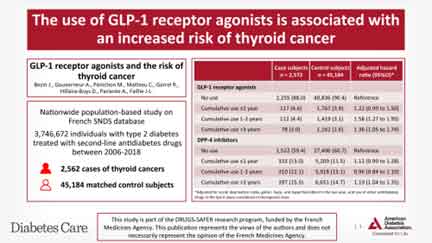
Bezin et al Diabetes Care Feb 2023
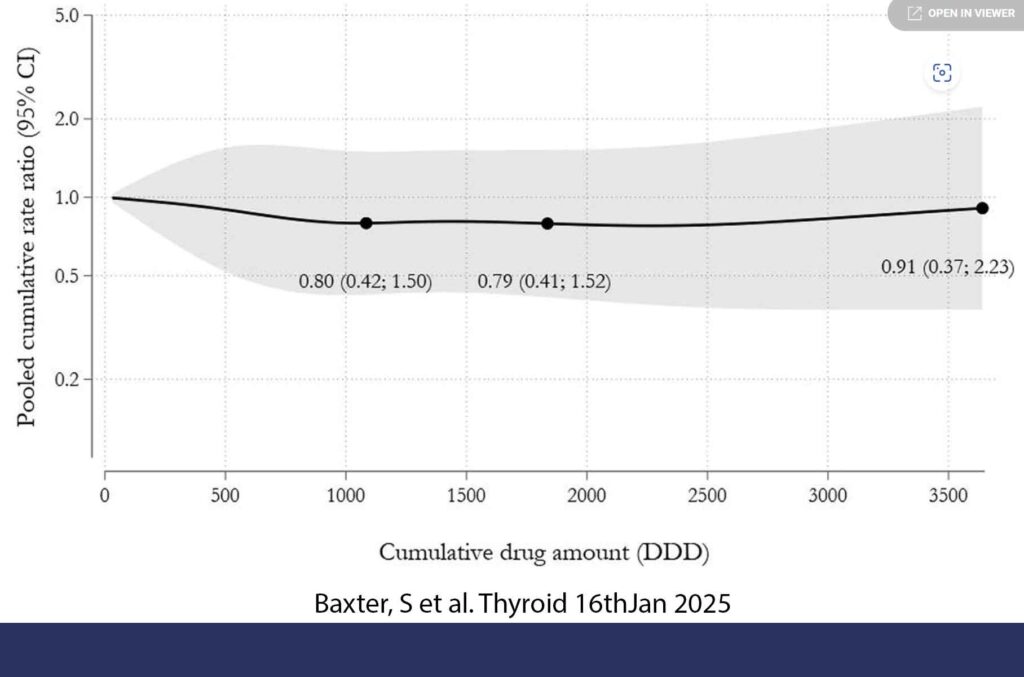
The study just published in Thyroid was a multisite cohort study with subsequent meta-analysis which included six population-based databases from Canada (Ontario), Denmark, Norway, South Korea, Sweden, and Taiwan (11). This was a big study. 98,147 users of GLP1-RA and 2,488,303 users of DPP-4i were followed up with the median follow-up among users of GLP1-RA ranging from 1.8 to 3.0 years. Overall, use of GLP1-RA compared with the use of DPP-4i was not associated with an increased risk of thyroid cancer (pooled weighted HR 0.81, CI 0.59–1.12). Similarly, there was no increased risk in thyroid cancer with increasing cumulative dose of GLP1-RA among GLP1-RA ever-users. See Figure 2. They tried to restrict the analysis to medullary cancer but the numbers were too low to be meaningful.
Interestingly, when they used users of sulphonylureas as the comparator, GLP1-RAs users had a significant elevation in risk of thyroid cancer (weighted HR 1.80, 95% CI 1.28–2.52). Now, prior research has found a positive association between weight and thyroid cancer risk (12). As GLP1-RAs are more likely to be prescribed in patients who has weight to lose, perhaps, we might be getting some confounding effect from obesity. The authors were unsure how well obese was recording in patients in the cohort.
This most recent study provide some reassurance that at least, in the short term (1-3 years), we can say that GLP1-RA is not associated with the risk of thyroid cancers. Because the numbers of medullary cancers are so small overall, we are unlikely to see any signals short term. There are certain groups of patients whom we would not be initiating a GLP1-RA in – patients with personal or family history of medullary cancer or multiple endocrine neoplasia syndrome type 2.
References:
- Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med2010;362:774-7.
- Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab. 2012 Jan;97(1):121-31
- Jung MJ, Kwon SK. Expression of glucagon-like Peptide-1 receptor in papillary thyroid carcinoma and its clinicopathologic significance. Endocrinol Metab (Seoul). 2014 Dec 29;29(4):536-44.
- Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, Jacobsen SD, Moses AC, Mølck AM, Nielsen HS, Nowak J, Solberg H, Thi TD, Zdravkovic M, Moerch U. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010 Apr;151(4):1473-86
- Madsen LW, Knauf JA, Gotfredsen C, Pilling A, Sjögren I, Andersen S, Andersen L, de Boer AS, Manova K, Barlas A, Vundavalli S, Nyborg NC, Knudsen LB, Moelck AM, Fagin JA. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012 Mar;153(3):1538-47.
- https://www.thyroidfoundation.org.au/Thyroid-Cancer. Accessed 22/2/2025
- Nauck MA, Jensen TJ, Rosenkilde C, Calanna S, Buse JB; LEADER Publication Committee on behalf of the LEADER Trial Investigators. Neoplasms Reported With Liraglutide or Placebo in People With Type 2 Diabetes: Results From the LEADER Randomized Trial. Diabetes Care. 2018 Aug;41(8):1663-1671
- Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract. 2012 Nov;98(2):271-84.
- Liang C, Bertoia ML, Ding Y, et al. Exenatide use and incidence of pancreatic and thyroid cancer: a retrospective cohort study. Diabetes Obes Metab 2019;21:1037–1042
- Bezin J, Gouverneur A, Pénichon M, Mathieu C, Garrel R, Hillaire-Buys D, Pariente A, Faillie JL. GLP-1 Receptor Agonists and the Risk of Thyroid Cancer. Diabetes Care. 2023 Feb 1;46(2):384-390
- Baxter SM, Lund LC, Andersen JH, et al. Glucagon-Like Peptide 1 Receptor Agonists and Risk of Thyroid Cancer: An International Multisite Cohort Study. Thyroid. 2025 Jan;35(1):69-78.
- Lauby-Secretan B, Scoccianti C, Loomis D, et al. International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer–viewpoint of the IARC working group. N Engl J Med 2016;375(8):794–798;
