23rd December 2024, A/Prof Chee L Khoo
Is this one of those “adverse events” related to the GLP1 agonist your patient saw on google one day? I can see you roll your eyes and I thought that way too initially. But upon further research, it seems to be a real thing as there was a study published in the JAMA Ophthalmology recently. We better look a bit more into the issue and the GLP1 agonists are here to stay. Non-arteritic anterior ischaemic optic nerve neuropathy (NAION) is a very significant cause of blindness amongst adults. Let’s dive into NAION and see what we have to look out for.
Anterior ischaemic optic neuropathy (AION) is defined as neuropathy involving the 1mm segment of the optic disc, and results in visible disc swelling. AION can either be non-arteritic (NAION) or arteritic (AAION) which is almost always associated with giant cell arteritis. There is another ischaemic optic neuropathy, posterior ischaemic optic neuropathy (PION), which involves the segment more posterior to the optic disc. PION does not appear with disc swelling.
The incidence of NAION is 2 to 10 cases per 100 000 persons making it the second most common cause of blindness due to optic nerve damage (with glaucoma being the most common) (1).
Symptoms and signs
NAION typically presents as an acute, monocular, painless loss of vision. Visual loss is usually less severe in NAION than in arteritic anterior ischemic optic neuropathy. Pain, headache or periocular pain is reported in 8-12% of patients. Dyschromatopsia (loss of colour vision) is a very sensitive sign of optic nerve dysfunction.
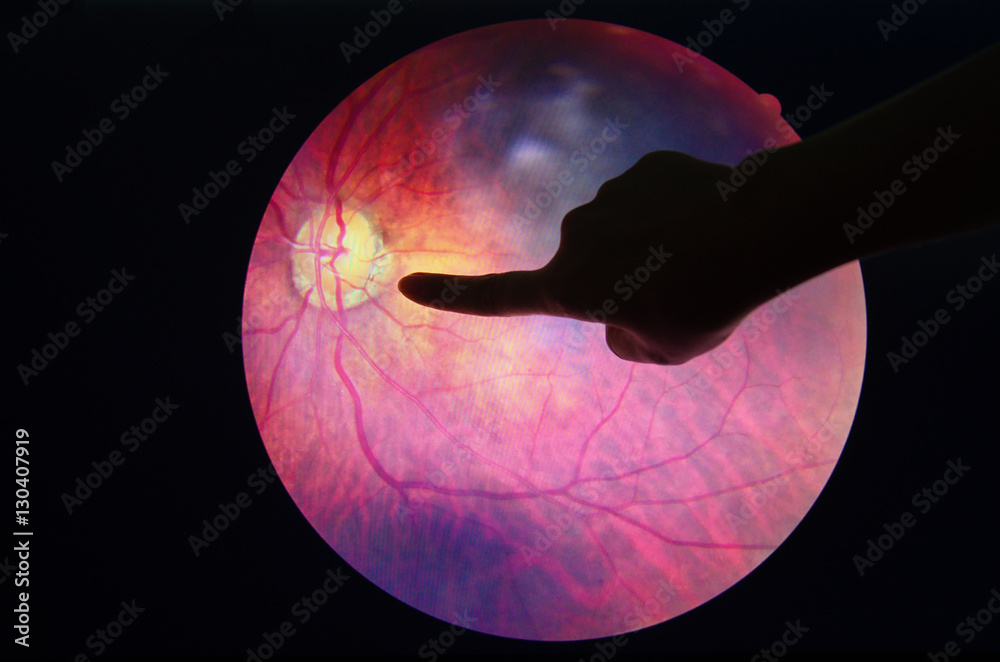
Funduscopic examination reveals diffuse or segmental optic disc oedema. Peripapillary splinter or flame haemorrhage, dilated telangiectatic capillaries, and narrowing of the peripapillary retinal arterioles can be observed in NAION. See Figure 1.
Pathophysiology of NAION
It is presumed to result from a circulatory insufficiency, or infarct, within the retrolaminar portion of the optic nerve head that is supplied by the short posterior ciliary arteries (SPCA). Fluorescein and indocyanine studies have shown delayed optic disc filling in the prelaminar layers of the optic disc with normal choroidal circulation (2,3). Optical coherence tomography angiography (OCTA) confirmed the presence of microvascular changes in cases of patients with acute optic disc oedema (4,5). It is thought that axonal oedema leads to crowding at the optic disc resulting in retinal ganglion death.
Aetiology and risk factors
Sleep apnoea
A case-control study of 17 consecutive patients with NAION found that 71% of the patients with NAION met criteria for SAS compared to 18% of controls (6). Retrospective cohort studies looking at national health insurance databases have found a significantly higher risk of NAION development in patients with SAS, even after controlling for confounding variables (7,8). It is thought that hypertension, raised intracranial pressure and nocturnal hypoxaemia leads to optic nerve ischaemia and oedema.
In the Ischemic Optic Neuropathy Decompression Trial (IONDT) where optic nerve decompression surgery were used to treat NAION, 60% of patients had at least one vasculopathic risk factor with hypertension (47%) and diabetes (24%) being most common [9]. Smoking does not seem to be an independent risk factor [10].
Drugs
Purvin (1995) reported the development of acute bilateral, sequential vision loss, likely from NAION, in 2 patients taking interferon alpha for malignant neoplasms [11]. Since then, there have been a handful of additional reports of a possible association (12-14). It is postulated that interferon alpha might cause an NAION by depositing immune complexes in the optic disc circulation that leads to ischemia.
Several prospective studies have shown increased risk of NAION development in patients with exposure to PDE5-inhibitors including sildenafil (15,16). It has been hypothesised that these medications might exaggerate the physiologic nocturnal hypotension resulting in ischemia to the optic nerve head and compartment syndrome in susceptible patients with small cup to disk ratios [17].
Semaglutide
In a retrospective matched cohort study using data from a centralised data registry of patients evaluated by neuro-ophthalmologists at Massachusetts Eye and Ear, Boston from December 1, 2017, through November 30, 2023, patients confirmed to have NAION and prescribed semaglutide were compared with those not prescribed semaglutide (18). Patients who had T2D and overweight or obese were analysed separately. They also assessed non-GLP1 weight loss medications bupropion, naltrexone, orlistat, topiramate, phentermine, and setmelanotide.
There were 16 827 patients for analysis. Patients who had T2D and overweight or obese were analysed separately.
Incidence of NAION in Patients With T2D
710 patients had T2D. The median age in this cohort was 57 (49-63) years for the semaglutide cohort and 58 (47-66) years for the non-semaglutide cohort. NAION occurred in 17 patients in the semaglutide cohort vs 6 in the comparative cohort. The Kaplan-Meier survival analysis at 36 months showed a cumulative incidence of NAION of 8.9% (95% CI, 4.5%-13.1%) for the semaglutide cohort vs 1.8% (95% CI, 0%-3.5%) for the non-semaglutide cohort. The Cox proportional hazards regression model showed a higher NAION risk in the semaglutide cohort vs the nonsemaglutide cohort (HR, 4.28;95%CI, 1.62-11.29; P < .001) Most of the incidence of NAION occurred in the first 12 months with cumulative incidence of 6.5% at 12 months.
Incidence of NAION in Patients Who Were Overweight or Obese
979 patients were classed as overweight or obese. The median (IQR) age was 46 (35-58) years for the semaglutide cohort and 44 (29-59) years for the non-semaglutide cohort. The Kaplan-Meier survival analysis at 36 months showed a cumulative incidence of NAION of 6.7% (95% CI, 3.6%-9.7%) for the semaglutide cohort vs 0.8% (95% CI, 0%-1.8%) for the non-semaglutide cohort. The Cox proportional hazards regression model showed a higher NAION risk in the semaglutide cohort vs the non-semaglutide cohort (HR, 7.64; 95% CI, 2.21-26.36; P < .001; concordance correlation coefficient = 0.86). Similar to the T2S cohort, most of the incidence of NAION occurred in the first 12 months with the cumulative incidence of 5.5% by 12 months.
In summary, this retrospective, matched cohort study appears to show that semaglutide use in patients with T2D and overweight or obese is very strongly associated with NAION (HR 4.28 and 7.64 respectively) compared with matched patients with T2D and overweight/obesity. Importantly, most of the incidence occurred within the first 12 months. We don’t know the mechanisms in this association yet.
While the general incidence of NAION is not high, the end result can be quite devastating for patients. Second eye involvement occurs in approximately 15% to 20% of patients with NAION within 5 years (8,9) and often results in a dramatic reduction in patient independence and quality of life. No therapy for acute NAION or prevention of fellow eye involvement has yet proved to be effective. In the IONDT trial, surgical decompression did not improve vision. Many pharmacotherapeutic agents have been found but to date no effective treatment has been found.
In a recent retrospective observational study, semaglutide treatment may be associated with more than a fourfold increase in the risk for NAION (19). However, the numbers were very small. The estimated absolute increase in the risk for NAION with GLP-1 therapy was 2.6 per 100,000 patient-years. In this meta-analysis, GLP-1 treatment was not associated with an increased risk for NAION (odds ratio, 1.53; 95% CI, 0.53-4.44; P = .43).
Nonetheless, in primary care, an acute visual loss demands an urgent referral to the ophthalmologist.
References
- Hattenhauer MGLeavitt JAHodge DOGrill RGray DT Incidence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol 1997;123 (1) 103- 107
- Arnold AC, Hepler RS. Fluorescein angiography in acute nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. Feb 15 1994;117(2):222-230.
- Oto S, Yilmaz G, Cakmakci S, Aydin P. Indocyanine green and fluorescein angiography in nonarteritic anterior ischemic optic neuropathy. Retina. Apr 2002;22(2):187-191.
- Balducci N, Morara M, Veronese C, et al. Optical coherence tomography angiography in acute arteritic and non-arteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2017;255(11):2255-2261
- Rougier MB, Delyfer MN, Korobelnik JF. OCT angiography of acute non-arteritic anterior ischemic optic neuropathy. J Fr Ophtalmol. 2017;40(2):102-109
- Mojon DS, Hedges TR, 3rd, Ehrenberg B, et al. Association between sleep apnea syndrome and nonarteritic anterior ischemic optic neuropathy. Arch Ophthalmol. May 2002;120(5):601-605.
- Sun MH, Lee CY, Liao YJ, Sun CC. Nonarteritic anterior ischaemic optic neuropathy and its association with obstructive sleep apnoea: a health insurance database study. Acta Ophthalmol. 2019;97(1):e64-e70
- Yang HK, Park SJ, Byun SJ, Park KH, Kim JW, Hwang JM. Obstructive sleep apnoea and increased risk of non-arteritic anterior ischaemic optic neuropathy. The British journal of ophthalmology. 2018
- Newman NJ. The Ischemic Optic Neuropathy Decompression Trial. Arch Ophthalmol. 2007;125(11):1568–1570. doi:10.1001/archopht.125.11.156
- Hayreh SS, Jonas JB, Zimmerman MB. Nonarteritic anterior ischemic optic neuropathy and tobacco smoking. Ophthalmology. Apr 2007;114(4):804-809
- Purvin VA. Anterior ischemic optic neuropathy secondary to interferon alfa. Arch Ophthalmol. Aug 1995;113(8):1041-1044.
- Willson RA. Visual side effects of pegylated interferon during therapy for chronic hepatitis C infection. J Clin Gastroenterol. Sep 2004;38(8):717-722.
- Rodney AJ, Gombos DS, Pagliaro LC, Tannir NM. Ischemic optic neuropathy associated with low-dose interferon alfa: report of two cases. Am J Clin Oncol. Feb 2009;32(1):86-87.
- Wei YH, Wang IH, Woung LC, Jou JR. Anterior ischemic optic neuropathy associated with pegylated interferon therapy for chronic hepatitis C. Ocul Immunol Inflamm. May-Jun 2009;17(3):191-194
- Aftab AM, Iqbal M, Rauf A, Ali A. Non arteritic anterior ischemic optic neuropathy; does Anticoagulation help?. J Ayub Med Coll Abbottabad. 2016;28(4):776-780
- Flahavan EM, Li H, Gupte-singh K, et al. Prospective Case-crossover Study Investigating the Possible Association Between Nonarteritic Anterior Ischemic Optic Neuropathy and Phosphodiesterase Type 5 Inhibitor Exposure. Urology. 2017;105:76-84
- Danesh-Meyer HV, Levin LA. Erectile dysfunction drugs and risk of anterior ischaemic optic neuropathy: casual or causal association? Br J Ophthalmol. Nov 2007;91(11):1551-1555
- Hathaway JT, Shah MP, Hathaway DB, Zekavat SM, Krasniqi D, Gittinger JW Jr, Cestari D, Mallery R, Abbasi B, Bouffard M, Chwalisz BK, Estrela T, Rizzo JF 3rd. Risk of Nonarteritic Anterior Ischemic Optic Neuropathy in Patients Prescribed Semaglutide. JAMA Ophthalmol. 2024 Aug 1;142(8):732-739. doi: 10.1001/jamaophthalmol.2024.2296.
- Silverii GA, Pala L, Cresci B, Mannucci E. Glucagon-like peptide 1 (GLP1) receptor agonists and risk for ischemic optic neuropathy: A meta-analysis of randomised controlled trials. Diabetes Obes Metab. 2024 Nov 20. doi: 10.1111/dom.16076.
