May 15, 2018, Dr Chee L Khoo
Unless you have experienced a severe hypoglycaemia, you don’t actually appreciate the petrifying effects it has on your patients. In addition to increased morbidity and mortality, it is associated with a reduction in health-related quality of life, increased fear and anxiety, reduced productivity and increased healthcare costs through increased utilisation of healthcare resources and blood glucose monitoring. As a result, patients may take compensatory actions in order to avoid hypoglycaemia, such as reducing their insulin dose, reducing exercise or increasing their calorie intake which can lead to poorer glycaemic control. It remains one of the major barrier to getting our patients to their glycaemic targets.
In a recent global, 4-week prospective study of hypoglycaemia in clinical practice conducted across 204 centres in 24 countries, a total of 8022 type 1 diabetes; 19,563 type 2 diabetes on insulin therapy were enrolled and surveyed to evaluate their knowledge and perceptions of hypoglycaemia over the previous 4 weeks. Increased blood glucose monitoring (69.7%) and seeking medical assistance (62.0%) were the most common responses in the 4 weeks following hypoglycaemic events for patients with type 1 diabetes and type 2 diabetes, respectively. Approximately 44% of patients with type 1 diabetes or type 2 diabetes increased calorie intake in response to a hypoglycaemic episode. Following hypoglycaemia, 3.9% (type 1 diabetes) and 6.2% (type 2 diabetes) of patients took leave from work or study.
Hypoglycaemia has a major impact on patients and their behaviour. These global data for the first time reveal regional variations in response to hypoglycaemia and highlight the importance of patient education and management strategies.
We know that insulin glargine (Lantus) have a relatively flat pharmacodynamic profile and the incidence of hypoglycaemia is a relatively low. From April 1, 2018, insulin glargine 300 (Toujeo®) is listed on the PBS. The new insulin glargine 300 units/ml (Gla‐300) provides the same insulin dose as your normal insulin glargine (Gla‐100) also known as Lantus® but in a third of the volume; it is hypothesised that this modifies the surface area of the subcutaneous depot influencing the redissolution rate. Gla‐300 appears to have better pharmacokinetic (PK) and pharmacodynamic (PD) profiles
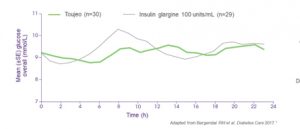
The PK/PD properties of Gla‐300 were evaluated in a series of randomized, double‐blind studies conducted in individuals with type 1 diabetes. The EDITION series of clinical trials was a worldwide comprehensive series of phase III trials that evaluated the efficacy and safety of Gla‐300 in diverse populations of people with diabetes. These studies in various populations of individuals with diabetes have consistently shown that Gla‐300 achieves similar HbA1c control, with a similar or lower incidence of confirmed or severe nocturnal hypoglycaemia, compared with Gla‐100 over 6 and 12 months.
The EDITION studies show mixed results regarding weight gain with Gla‐300 and Gla‐100 over 6 months; there was significantly less weight gain in some of the studies and no significant difference in weight gain in others.
The findings of the phase III, EDITION series of clinical trials suggest that Gla‐300 could be a viable treatment option for a range of people with type 1 and type 2 diabetes. Gla‐300 was shown to result in similar glycaemic control to Gla‐100 in individuals with type 2 diabetes who were taking basal and mealtime insulins. Confirmed or severe nocturnal hypoglycaemia was generally lower with Gla‐300 compared with Gla‐100, although the incidence of confirmed or severe hypoglycaemia at any time of day was equal or lower.
Reference
- Dailey G, Lavernia F. A review of the safety and efficacy data for insulin glargine 300 units/ml, a new formulation of insulin glargine. Diabetes Obes Metab. 2015 Dec;17(12):1107-14
- Khunti R, et al. Impact of hypoglycaemia on patient-reported outcomes from a global, 24-country study of 27,585 people with type 1 and insulin-treated type 2 diabetes. Diabetes Reaserch and Clinical Practice 1 3 0 ( 2 0 1 7 ) 1 2 1 –1 2 9
- Bergenstal RM et al. Diabetes Care 2017; 40(4):554–60.
