23rd March 2019, Dr Chee L Khoo
American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) suggest that if HbA1c targets are not achieved despite the addition of basal insulin in type 2 diabetes (T2D), treatment could also be further advanced by the addition of a glucagon-like peptide-1 receptor agonist (GLP-1 RA). The addition of a GLP1-RA has been shown to be as efficacious as the addition of a prandial insulin in patients with T2D who is not at glycaemic target despite the addition of basal insulin therapy (1). It has further advantages of lower weight gain (or actual weight loss) and lower rates of hypoglycaemia compared with a basal + bolus insulin therapy.
What is better than a basal insulin plus GLP1-RA regimen? It would be nice if they both come in combination pen device, wouldn’t it? Indeed, we do have one coming very soon to the Australian market. Soliqua® is a combination of insulin glargine (U100 – i.e. our good old lantus) and lixisenatide.
Lixisenatide is a GLP1- receptor agonist which targets fasting plasma glucose (FPG) and post-prandial plasma glucose to improve glycaemic control in patients with T2D, while minimising weight gain and risk for hypoglycaemia. It acts by stimulating insulin secretion (but only in hyperglycaemia) and suppressing glucagon secretion. It also slows gastric emptying thereby reducing the rate at which meal-derived glucose is absorbed and appears in the circulation. Thus, lixisentatide looks after the post prandial glucose rise.
It makes sense to combine a basal insulin with a GLP1-RA as insulin glargine provides the background and overnight insulin needs while lixisenatide looks after the post prandial needs. Does it work in clinical practice? The safety and effectiveness of Soliqua® on glycaemic control were evaluated in two randomised clinical studies in patients with type 2 diabetes mellitus.
In the LixiLan-O study, Soliqua® was added to metformin in patients with T2D who were not adequately controlled on metformin and another oral anti-diabetic drug (OAD). Their HbA1c was between 7.5-10.0%. During the 4 week run-in phase, metformin was maximised and the second OAD was stopped. After the run-in phase, 1170 patients were randomised to either Soliqua®, insulin glargine alone or lixisenatide alone. The mean HbA1c was 8.1% at baseline. The average duration of diabetes in these patients were 9 years.
At the end of the study of 30 weeks, Soliqua® reduced fasting, postprandial and HbA1c more than insulin glargine alone or lixisenatide alone – see Table 1. Mean body weight was -0.3kg, + 1.1kg and -2.3kg in Soliqua®, glargine and lixisenatide groups respectively. In other words, Soliqua® reduced the weight gain often associated with insulin therapy.
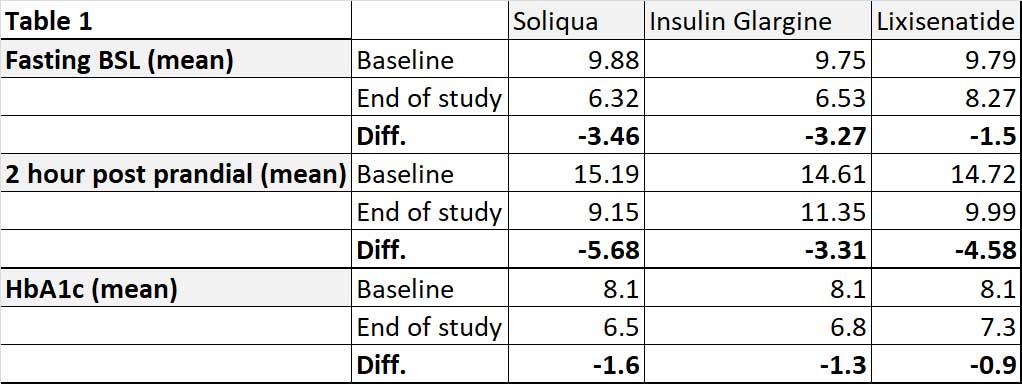
In the LixiLan-L study, 760 patients with T2D stabilised on basal insulin for at least 6 months with doses between 15-40 units and still had HbA1c of between 7.5% – 10.0%. They may be on one or more OADs. In the 6 week run-in phase, metformin was continued but the other OAD were discontinued. After the run-in phase, patients were either continued on insulin glargine or switched to Soliqua®. These patients, as expected, had diabetes for longer duration (12 years). At the end of 30 weeks of study, patients on Soliqua® had better HbA1c (6.9 vs 7.5%) and better post prandial glucose (9.91 vs 13.41 mmol/L) when compared with insulin glargine alone.
Overall, the hypoglycaemic events were low. The corresponding number of events per patient-year was lower in the Soliqua® group than in the insulin glargine group (3.03 vs. 4.22). Patients on Soliqua® on average lost 0.7kg of body weight from baseline compared to 0.7kg weight gain in the insulin glargine group a 1.4 kg difference between groups. 54% patients on Soliqua® did not gain weight while 62% of patients on insulin glargine gain weight during the study period. More patients on Soliqua® achieved HbA1c of < 7.0% without weight gain or hypoglycaemia compared with insulin glargine alone (20.8% vs 13.2%, p<0.0001). In other words, patients achieved glycaemic control with the side effects of weight gain or hypoglycaemia with Soliqua®. GI side effects were low in both studies.
Soliqua® represent another valuable treatment option for patients with T2D whose glycaemic control is still suboptimal with oral therapy or basal insulin. It comes in a convenient two-in-one pen device. It could be useful in patients reasonably early in their diabetes career or elderly patients whose HbA1c is still high while on basal insulin alone. The GLP1-RA component in Soliqua® might be enough to achieve better glycaemic control with a low risk of hypoglycaemia.
Soliqua® is coming soon to Australia. It is before the PBAC for consideration as I write this article.
References:
- Rosenstock J, Guerci B, Hanefeld M, et al.; GetGoal Duo-2 Trial Investigators. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 trial. Diabetes Care 2016;39:1318–1328
- Julio Rosenstock, Ronnie Aronson, George Grunberge et al. Benefits of LixiLan, a Titratable Fixed-Ratio Combination of Insulin Glargine Plus Lixisenatide, Versus Insulin Glargine and Lixisenatide Monocomponents in Type 2 Diabetes Inadequately Controlled on Oral Agents: The LixiLan-O Randomized Trial. Diabetes Care 2016 Nov; 39(11): 2026-2035
- Vanita R. Aroda, Julio Rosenstock, Carol Wysham, et al. Efficacy and Safety of LixiLan, a Titratable Fixed-Ratio Combination of Insulin Glargine Plus Lixisenatide in Type 2 Diabetes Inadequately Controlled on Basal Insulin and Metformin: The LixiLan-L Randomized Trial. Diabetes Care 2016 Sep; dc161495
