14th March 2020, Dr Chee L Khoo
Despite significant reduction in cardiovascular events over the last 20 years in the general population, patients with diabetes still has 1.5-2 times the risk of cardiovascular events and cardiovascular mortality compared with the general population. It was pretty exciting when two classes of the new anti-diabetic medications have been shown in a number of landmark trials to reduce composite cardiovascular events patients with type 2 diabetes (T2D) and previous cardiovascular disease or patients with T2D with multiple cardiovascular risks.
Remember, the cardiovascular outcome trials (CVOTs) were originally designed to demonstrate the safety of new anti-diabetic drugs following the worries from adverse reports on rosiglitazone (Avandia) and following the surprising results from the ACCORD study which showed increase mortality with intensive glucose lowering in T2D patients with high cardiac risks. To demonstrate cardiovascular safety, all that was needed was for drugs to demonstrate composite major adverse cardiovascular events (MACE) that are not higher than placebo. MACE consisted of non-fatal MI, non-fatal stroke and cardiovascular mortality. Much to our surprise, not only did many of the agents demonstrated cardiovascular safety, some demonstrated cardiovascular benefits. The SGLT2 inhibitors and GLP-1 agonists not only improve glycaemic control, both classes have been shown to improve MACE in patients with T2D and existing cardiovascular disease.
We looked at the 2019 AHA/EASD hyperglycaemia management guidelines couple of months ago here. There were a number of minor yet significant updates from the 2018 guidelines. We highlighted 5 recommendations arising out of the guidelines. Recommendation 1 – T2D with atherosclerotic CV disease (ASCVD) is worth exploring in detail. This recommendation was based on the findings from the REWIND study which published in June 2019. Let’s look at the study in detail and see how it relates to our management of patients with type 2 diabetes in general practice.
Dulaglutide comprises of two GLP-1 molecules linked together and have a half life of 5 days. It is given subcutaneously weekly. Like other GLP-1 agonists, it reduces glucose, blood pressure and body weight. CVOTs from other GLP-1 agonists (shows cardiovascular benefits with reduction in the composite MACE in patients with high cardiovascular risks. The Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) trial was designed to assess whether the addition of dulaglutide to the diabetes medication regimen of middle-aged and older people with type 2 diabetes safely reduces the incidence of cardiovascular outcomes compared with placebo.
Patients with T2D aged >50 years old whose HbA1c ≤9.5% on up to two oral antidiabetic agents with or without basal insulin were randomised in a double-blind placebo controlled multi-centre trial. The patients need to have vascular disease (ie, a previous myocardial infarction, ischaemic stroke, revascularisation, hospital admission for unstable angina, or imaging evidence of myocardial ischaemia. Patients > 55 years old had to have myocardial ischaemia, coronary, carotid, or lower extremity artery stenosis exceeding 50%, left ventricular hypertrophy, estimated glomerular filtration rate (eGFR) less than 60 mL/min per 1・73 m2, or albuminuria; and those aged 60 years or older had to have at least two of tobacco use, dyslipidaemia, hypertension, or abdominal obesity. In other words, if you look at the patient mix in this study, you will note that the mix is similar to the patient mix that we see at our practice – some with established cardiovascular disease but many have a few cardiovascular risk factors.
The primary outcome was the occurrence of any component of MACE. The secondary outcomes were a composite of progression of retinopathy, renal disease and hospitalisation for unstable angina.
The results
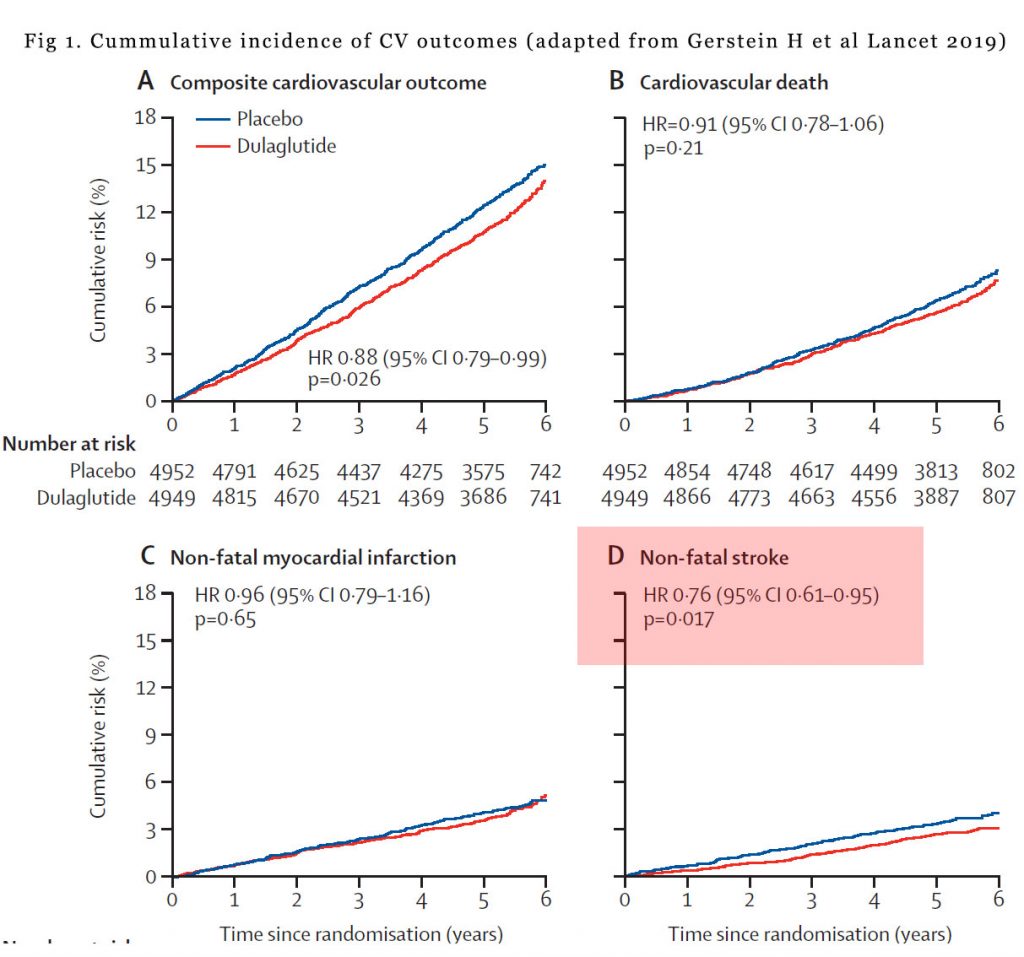
All up 9901 patients were randomised to either weekly dulaglutide or placebo. Average age of the patients were 66.2 years, average duration of diabetes was 9.5 years. 31.5% of subjects had previous cardiovascular disease and 22% had an eGFR of ≤ 60 mL/min per 1・73 m2. After an average of 5.4 years of follow up, the primary composite MACE occurred in 12.0 % of patients on dulaglutide and 13.4% of patients on placebo (hazard ratio of 0.88, p=0.026). However, when you break down the individual components of the MACE, the hazard ratio (HR) for cardiovascular death was 0.91 (p=0.21) and for non-fatal myocardial infarction was 0.96 (p=0.65) which is not statistically significant. Only the HR for non-fatal stroke of 0.76 (p=0.17 was statistically significant. In other words, much of the composite MACE outcomes came from improvement in non-fatal stroke. See Figure 1.
When the data was sub-analysed, the HR were unchanged whether the subjects had prior cardiovascular disease or not or whether the HbA1c was above 7.2% or below 7.2%. The HR were also unaffected by duration of diabetes, age, sex or BMI.
There was a reduction in the composite microvascular outcomes (HR 0.87) primarily driven by reduction in the composite renal events (HR 0.85). There were no reduction in all-cause mortality, heart failure, revascularisation or hospital admissions. As expected, dulaglutide resulted in lower HbA1c (-0.61%), body weight (-1.46kg), systolic BP (-1.7mmHg), higher heart rate (+1.87 beats per min), lower total cholesterol (-0.07 mmol/L) and lower LDL (-0.05 mmol/L).
In patients with T2D and multiple risk factors, the number to treat (NTT) to prevent one cardiovascular event was 60 but for patients with T2D with known previous cardiovascular disease, the NTT was 18.
In summary
The addition of weekly dulaglutide to existing anti-diabetic agents in patients with T2D with a range of HbA1c can reduced cardiovascular events although most of the benefits were in the reduction of non-fatal strokes. This benefit was seen in both patients with previous cardiovascular events or with multiple cardiovascular risks. Further, dulaglutide can also reduce microvascular events especially renal events. These improvements in macro and microvascular outcomes comes on top of improvement in HbA1c , body weight, systolic BP and lipids.
As the 2019 ADA/EASD hyperglycaemia managmenet guidelines indicated, when managing hyperglycaemia in patients with T2D, one should consider more than just glycaemic control. There are agents which has other benefits in addition to reduction in HbA1c. In CVOTs, both the SGLT2 inhibitors and GLP-1 agonists have been shown to reduce composite MACE. However, much of the benefits in the SGLT2 inhibitors class came from reduction in hospitalisation for heart failure. The REWIND study shows that dulaglutide appears to have more stroke prevention benefits. Both classes appear to show renal benefits.
Reference:
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled Trial. Lancet 2019; 394: 121–30
