12th June 2020, Dr Chee L Khoo
Like me, you probably have come across homocysteine, methionine and MTHFR gene polymorphism from time to time and are aware of some connection between those things and cardiovascular disease but not quite sure how to connect them together. Which patient should we be checking homocysteine levels in? What about the MTHFR gene polymorphism? How does that fit into the whole jigsaw? You are also probably not sure what to do when you do find elevated homocysteine levels in a patient.
We better start from basics again. Let’s look at the whole theory behind where homocysteine fits into clinical practice. Then we can see what the evidence says. We can then look at whether there is any treatment for the problem.
Homocysteine and Methionine metabolism
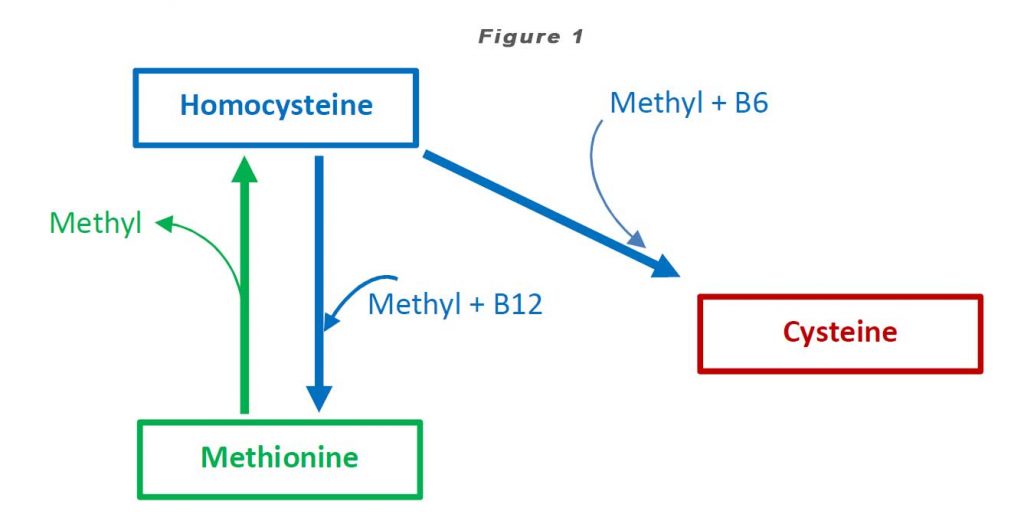
Homocysteine is a homologue of the amino acid, cysteine (“homo-cysteine”), differing by the addition of a methylene bridge (-CH2-). Homocysteine is synthesized from methionine by removal of the terminal methyl group. Homocysteine can be recycled back to methionine or cysteine with the aid of Vitamin B12 and Vitamin B6 respectively (see Figure 1).
High homocysteine levels (HHcy) promotes vascular disease, including endothelial dysfunction, smooth muscle cell proliferation, and cardiovascular remodelling [1]. On the basis of investigations in mice and rats, it was found that HHcy also induces a cardiac metabolic disorder due to oxidative stress-diminished cardiac O2 consumption, which may lead to cardiac metabolic disease [2]. Recently, it has been reported that Hcy is a potential marker for severe disease in Covid-19 (3).
Methionine (Met) is an essential amino acid in humans. It cannot be synthesized de novo and is found in eggs, meat, fish, brazil nuts and cereal grains. It is a substrate for other amino acids such as cysteine and taurine and the important anti-oxidant, glutathione. Met plays a critical role in the metabolism and health in humans.
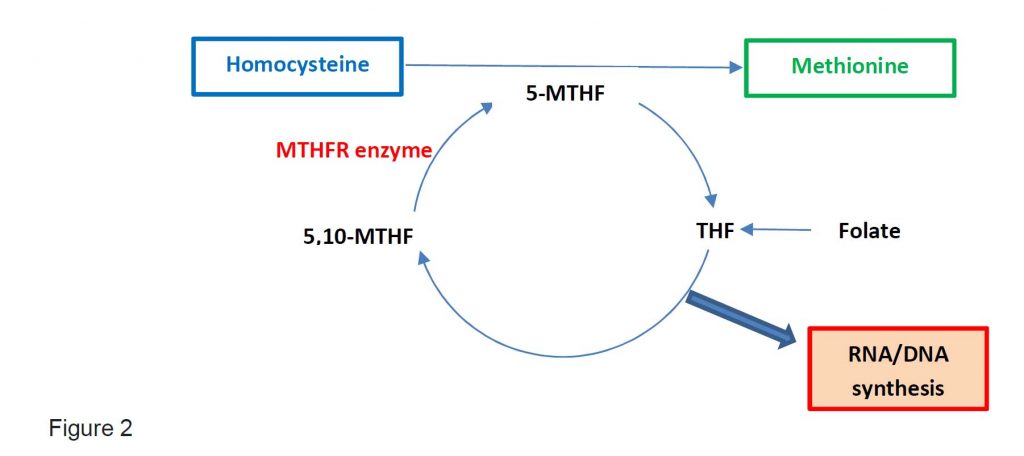
Folate exist as tetrahydrofolate (THR) and is the substrate for 5,10-methylene-THF which is converted into 5-methyl-THF by MTHFR enzyme. 5-methyl-THF is required when Hcy is converted into Met. Thus, folate deficiency will cause an increase in Hcy levels.
Where does MTHFR gene polymorphism fit in all these?
The MTHFR enzyme is encoded by the MTHFR gene. In the MTHFR gene, the MTHFR nucleotide at position 677 in the gene is normally cytosine (C) but may be replaced by thymine (T) in some individuals. Two copies of 677C (677CC) is the most common allele. Individuals with both copies of 677T (677TT) have lower MTHFR activity and have been associated with lower folate and higher homocysteine levels compared with individuals with 677CC or 677CT allele. Individuals with the 677TT genotype had approximately 30% of the enzyme activity in those with the 677CC genotype, and the heterozygotes 677C/T had around 65% of the latter.
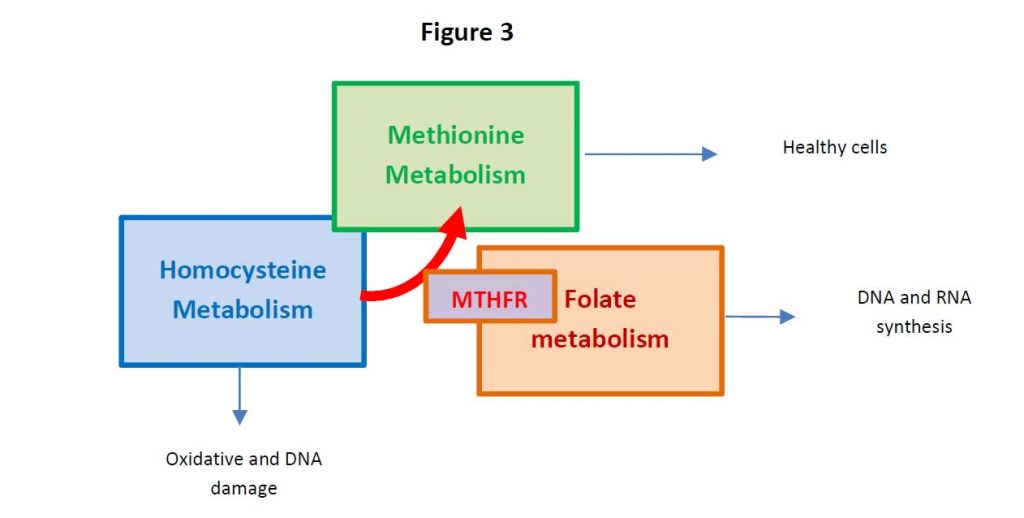
One carbon metabolism is the metabolism which generates thymidine and other purines in the DNA and RNA synthesis (see Figure 2).
How does all these fit into health and diseases in practice?
Persistent HHCys promotes the formation of atherosclerotic plaques, atherothrombotic events through endothelial dysfunction, the enhancement of inflammation and the so-called thrombophilic profile (3). For these reasons, in addition to the traditional risk factors, both the World Health Organization (WHO) considers HHCys a strong contributor for cardiovascular disease (4).
The Swedish National Study on Aging and Care in Kungsholmen is a cohort study of randomly selected individuals aged ≥ 60 years to investigate the association of serum homocysteine and methionine with the rate of CV multi-morbidity development in older adults and to explore the role MTHFR) 677C>T gene in this association (5). The CV disease they looked for included ischemic heart disease, stroke, heart failure, atrial fibrillation, cardiac valve diseases, bradycardias and conduction disorders and peripheral artery disease.
A higher concentration of Hcy, a lower concentration of Met, and a lower Met:Hcy ratio (suggesting impaired methylation activity) were all associated with an increased rate of CV multimorbidity development during a 12-year period. Genetic predisposition (ie, the MTHFR 677T allele) further contributed to accelerated CV multimorbidity development in participants with low Met concentrations. Results were independent of sociodemographic, lifestyle, and clinical factors.
Does treatment help?
You would expect that because high Hcy is associated with increased cardiovascular events and that because vitamin B (folate, B6 and B12) are integral to the metabolism of Hcy and methionine, reducing Hcy with vitamin B will reduce cardiovascular events. However, the evidence is conflicting.
In the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST), high dose folic acid reduced Hcy by 19% but did not slow the progression of atherosclerosis or CV events in patients with CKD (6). In the Heart Outcomes Prevention Evaluation 2 (HOPE-2) trial, 2764 patients treated with high dose folate, vitamin B6 and vitamin B12 over 5 years reduced Hcy by 25% but did not reduce CV events even in patients with pre-existing CV disease or diabetes (7). Other trials, NORVIT (8) and WENBIT (9) show similar negative results.
The folate after coronary intervention trial (FACIT) in fact, showed that folic acid, vitamin B12 and B6 after coronary stenting might increase the risk of in-stent restenosis and the need for target-vessel revascularization (10).
When Bazzano et al performed a meta-analysis of 12 RCT which compared folic acid with placebo, they did not find reduction in risk of CV disease or all-cause mortality in patients with prior history of vascular disease (11). In the Vitamin Intervention for Stroke Prevention (VISP) trial indicated no benefit of B vitamins on the risk of myocardial infarction, whereas a subgroup analysis of the VISP trial showed effect in a defined subset of the population (12).
The effects of Hcy lowering by B vitamins might be dependent on the disease stage. In the absence of atherosclerotic lesions in any more or less advanced stage, the lower Hcy might have beneficial effects, whereas in subjects with significant atherosclerotic lesions, the B vitamins might have detrimental effects, neutralizing the benefit of Hcy lowering.
Who should we screen for high homocysteine levels?
American Heart Association, normal serum Hcy concentration range from 5 to 15 µmol/L, 15–31 µmol/L was considered mildly increased, and 31–100 µmol/L was regarded as intermediately elevated, while severely elevated was over 100 µmol/L, although 12 µmol/L was considered elevated by others.
So who should check Hcy in?
The majority of patients with CAD have the traditional CV risk factors but there is a significant number of patients who don’t. Some of those patients may have high Hcy. Thus, patients who have CAD especially at a younger age (under 40 years old) but do not have the traditional CV risk factors may have high Hcy. Similarly, patients who have family members with CAD at a younger age who also don’t have the traditional CV risk factors could have high Hcy. What about patients with chronic kidney disease but do not have risk factors for CKD? Are we missing high Hcy in these patients? The guidelines are not that clear as to whom we should screen for high homocysteine?
What about treatment?
Thus far, the trials have not shown that homocysteine lowering therapy using vitamin B (B6, 9,12) have reduced CV mortality.
Should we measure MTHFR gene?
Well, since the evidence isn’t there with homocysteine lowering, measuring MTHFR gene polymorphism is rather pointless at this stage. The guidelines definitely say no to testing for MTHFR gene polymorphism.
We are left with ensuring that all risk factors are attending to. In particular, very tight lipid control and regular reminders of lifestyle measures.
References:
- Steed MM, Tyagi SC. Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxid Redox Signal 2011;15:1927–1943.
- Becker JS, Adler A, Schneeberger A, et al. Hyperhomocysteinemia, a cardiac metabolic
- disease: role of nitric oxide and the p22phox subunit of NADPH oxidase. Circulation 2005; 111:2112–2118.
- Ponti G, Ruini C, Tomasi A. Homocysteine as a potential predictor of cardiovascular risk in patients with COVID-19. Medical Hypotheses Volume 143, October 2020, 109859. https://doi.org/10.1016/j.mehy.2020.109859
- World Health Organization. Prevention of Cardiovascular Disease Guidelines for Assessment and Management of Cardiovascular Risk WHO Library Cataloguing-in-Publication Data (2007). https://www.who.int/cardiovascular_diseases/guidelines/Full%20text.pdf
- Calderón-Larrañaga A, Saadeh M, Hooshmand B, et al. Association of Homocysteine, Methionine, and MTHFR 677C>T Polymorphism With Rate of Cardiovascular Multimorbidity Development in Older Adults in Sweden. JAMA Netw Open. 2020;3(5):e205316. Published 2020 May 1. doi:10.1001/jamanetworkopen.2020.5316
- Zoungas S, McGrath BP, Branley P, et al. Cardiovascular morbidity and mortality in the Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in chronic renal failure: a multicenter, randomized, controlled trial. J Am Coll Cardiol. 2006;47(6):1108‐1116. doi:10.1016/j.jacc.2005.10.064
- Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease [published correction appears in N Engl J Med. 2006 Aug 17;355(7):746]. N Engl J Med. 2006;354(15):1567‐1577. doi:10.1056/NEJMoa060900
- Bønaa KH, Njølstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578‐1588. doi:10.1056/NEJMoa055227
- Løland KH, Bleie O, Blix AJ, et al. Effect of homocysteine-lowering B vitamin treatment on angiographic progression of coronary artery disease: a Western Norway B Vitamin Intervention Trial (WENBIT) substudy. Am J Cardiol. 2010;105(11):1577‐1584. doi:10.1016/j.amjcard.2010.01.019
- Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369(9557):208‐216. doi:10.1016/S0140-6736(07)60109-3
- Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials [published correction appears in JAMA. 2007 Mar 7;297(9):952]. JAMA. 2006;296(22):2720‐2726. doi:10.1001/jama.296.22.2720
