12th June 2020, Dr Chee L Khoo

Whenever there are multiple brands of the same class of drugs on the market, one always wonder whether the new kid on the block is a “johnny-come-lately” trying to break into an already crowded market with yet another drug or it’s really a new kid with much better credentials. We already have 4 GLP-1 agonists in Australia and now a fifth one will be out real soon. How does the new kid compare with the rest of the kids? Is it a “johnny-come-lately” or a much improved kid of the class? We are referring to Semaglutide which is coming to showroom near you very soon.
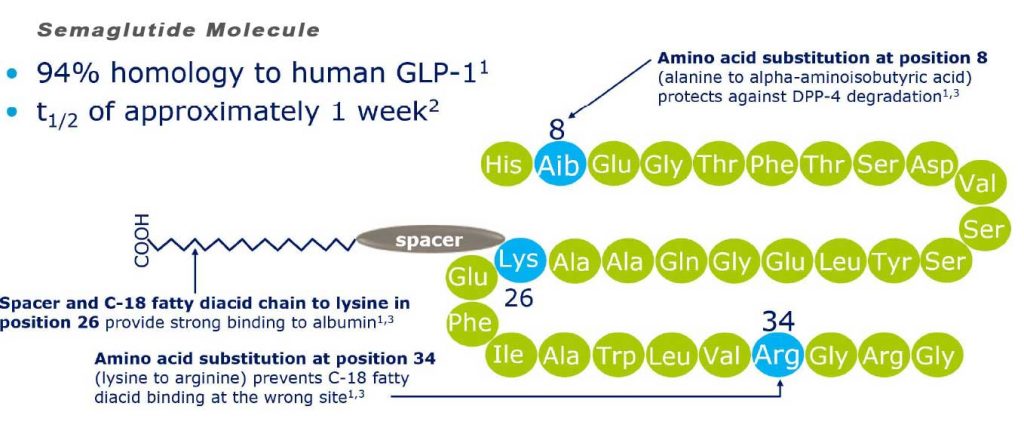
Semaglutide is chemically similar to human GLP-1 with 94% similarity. The only differences are two amino acid substitutions at positions 8 and 34, where alanine and lysine are replaced by 2-amino-isobutyric acid and arginine respectively.[11] Amino acid substitution at position 8 prevents chemical breakdown by an enzyme dipeptidyl peptidase-4. Acylation with a spacer and C-18 fatty diacid chain increases the drug binding to blood protein (albumin), which enables longer presence in the blood circulation.[12] Its half-life in the blood is about 7 days (165–184 hours), therefore, once-weekly injection is enough.
Like all other GLP-1 agonists, the mechanisms of action of semaglutide are:
- Decreased glucagon concentrations
- Improved insulin sensitivity
- Decreased A1C
- Slowed gastric emptying
- Increased satiety
- Decreased free fatty acid concentrations
- Decreased body weight
There are suggestions that GLP-1 agonists may have trophic effects on beta cells (i.e. increase beta cell mass).
So, how does semaglutide compare with other anti-diabetic agents?
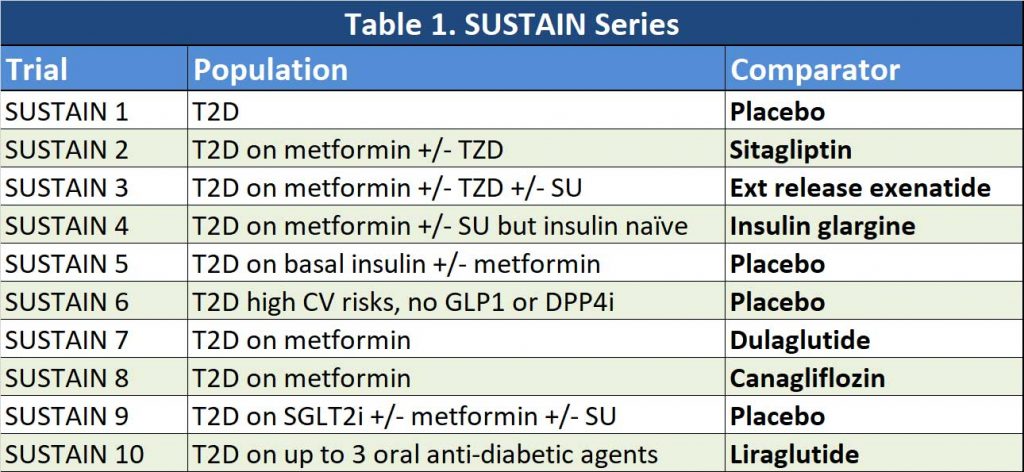
Semaglutide have been compared with a number of agents in a series of SUSTAIN trials. See Table 1. This represents a whole variety of patients we may encounter in our practice. So, how does it compare in a few practical scenarios:
Patients on metformin monotherapy – second line agent (SUSTAIN 7 and SUSTAIN 8)
SUSTAIN 7: These are patients with T2D uncontrolled on metformin only. Semaglutide 0.5mg weekly and semaglutide 1.0mg weekly were compared with Dulaglutide 0.75mg weekly and Dulaglutide 1.5mg weekly. Over a 40-week period, low dose Semaglutide was superior to low dose Dulaglutide (-1.5% vs -1.1%). Similarly, high dose Semaglutide was also superior to high dose dulaglutide (-1.8% vs -1.4%). Patients on low dose semaglutide lost more weight compared with low dose dulaglutide (-4.6kg vs -2.3kg). High dose semaglutide was also better than high dose dulaglutide with weight loss (-6.5kg vs -3.0kg).
SUSTAIN 8: These are patients with T2D uncontrolled on metformin only. Semaglutide 1.0mg was compared with Canagliflozin 300mg (an SGLT2 inhibitor not available in Australia). Over a follow up period of 52 weeks, semaglutide 1.0mg was significantly superior to canagliflozin 300mg in improving HbA1c (-1.5% vs -1.0%) and with weight loss (-5.2kg vs -4.2kg).
Patient on metformin and other orals
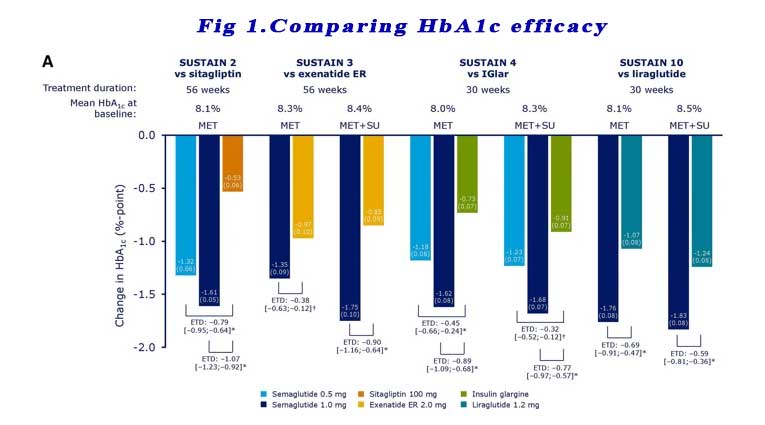
SUSTAIN 2, 3, 4,10: These trials compared Semaglutide in patients who are already on metformin and other oral anti-diabetic agents (TZD +/- SU +/- SGLT2i) to Sitagliptin, extended release exenatide, liraglutide and basal insulin. Significantly greater reductions in HbA1c from baseline were observed with subjects receiving semaglutide 0.5 mg or 1.0 mg vs comparators across all four trials at the end of treatment (week 56 for SUSTAIN 2 and 3 and week 30 for SUSTAIN 4 and 10). See Figure 1.
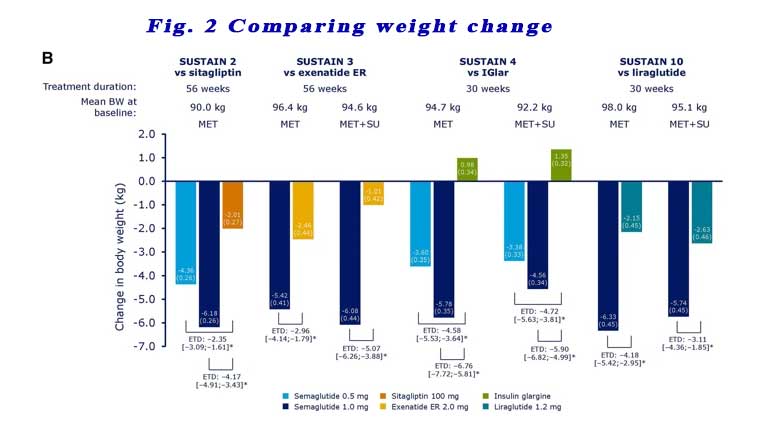
Reductions in body weight were also consistently statistically greater with semaglutide 0.5 mg and 1.0 mg vs comparators across all four trials, regardless of background OAD(s). See Figure 2.
Patient on basal insulin +/- metformin
SUSTAIN 5: These are patients with T2D uncontrolled on basal insulin +/- metformin. Semaglutide 0.5mg and 1.0mg were compared with placebo. Traditionally, these patients would be started either on a mixed insulin or a basal-bolus regimen. These days, we do have an alternative. We could add a GLP-1 agonist instead. In SUSTAIN 5, patients were receiving stable basal insulin for 90 days prior to randomisation. To limit the risk of hypoglycaemia, in patients whose HbA1c was ≤8% at baseline, their insulin doses were reduced by 20%.
At week 30, mean HbA1c values with semaglutide 0.5 and 1.0 mg were 6.9% and 6.5%, vs 8.3% with placebo. Significantly more patients achieved an HbA1c target of <7.0% with semaglutide 0.5 mg and 1.0 mg than with placebo (61%, 79%, and 11%) and an HbA1c target ≤6.5% with semaglutide 0.5 and 1.0 mg vs placebo (41%, 61%, and 5%).
For patients with HbA1c ≤8%, there were more hypoglycaemic episodes in the semaglutide groups compared with placebo. For patients with HbA1c >8%, the hypoglycaemic episodes were comparable between the groups. The total dose of insulin was reduced over the 30 weeks in all groups.
At week 30, mean body weight decreased with semaglutide 0.5 and 1.0 mg vs placebo by 3.7, 6.4, and 1.4 kg
Patient with high CV risks
SUSTAIN 6: These are patients with T2D with HbA1c ≥7.0% with establish cardiovascular disease or chronic kidney disease ≥ stage 3. Patients who have been on a DPP4 inhibitor or a GLP-1 agonist are excluded. They may be on insulin or metformin.
Now, you do remember that current management guidelines for the management of patients with T2D is less glucose centric. We need to consider (amongst other things) what that patient’s CV or renal risks are. So, it is appropriate that SUSTAIN 6 looked at patients with established CVD or CKD and major adverse cardiac events (MACE).
The trial was not looking at efficacy of HbA1c reduction or weight loss. Semaglutide 0.5mg and 1.0mg were compared with placebo over 104 weeks. The composite primary outcome was the first occurrence of major adverse cardiovascular events (cardiovascular death, nonfatal MI and nonfatal stroke).
Semaglutide treated patients had a significant 26% lower risk of the primary composite outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke than did those receiving placebo. This lower risk was principally driven by a significant (39%) decrease in the rate of nonfatal stroke and a nonsignificant (26%) decrease in nonfatal myocardial infarction, with no significant difference in the rate of cardiovascular death. Similar risk reductions were observed with both doses of semaglutide. Semaglutide-treated patients had a lower risk of new or worsening nephropathy, according to differences in macroalbuminuria.
In general, the prevalence of nausea events over time with semaglutide treatment was ;3% to 5% throughout the study.
Worsening of retinopathy
Although the overall number of retinopathy events was low, there was an unexpected higher rate of retinopathy complications (vitreous hemorrhage, blindness, or the need for treatment with an intravitreal agent or photocoagulation) in the semaglutide group. An association between rapid glucose lowering and worsening of retinopathy has been reported in patients with type 1 diabetes.9,10
In summary, the up and coming GLP-1 agonist, semaglutide will hit our shores very soon. It appears from the SUSTAIN series of trials to be more efficacious than other anti-diabetic agents (including other GLP-1 agonists) and results in better weight reduction. In addition, it has better cardiovascular and renal outcomes.
References
- Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275‐286. doi:10.1016/S2213-8587(18)30024-X
- McCrimmon RJ, Catarig AM, Frias JP, et al. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: a substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia. 2020;63(3):473‐485. doi:10.1007/s00125-019-05065-8
- Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial [published correction appears in Lancet Diabetes Endocrinol. 2019 Mar 11;:] [published correction appears in Lancet Diabetes Endocrinol. 2019 Aug;7(8):e20] [published correction appears in Lancet Diabetes Endocrinol. 2019 Nov;7(11):e22]. Lancet Diabetes Endocrinol. 2019;7(5):356‐367. doi:10.1016/S2213-8587(19)30066-X
- Rodbard HW, Lingvay I, Reed J, et al. Semaglutide Added to Basal Insulin in Type 2 Diabetes (SUSTAIN 5): A Randomized, Controlled Trial. J Clin Endocrinol Metab. 2018;103(6):2291‐2301. doi:10.1210/jc.2018-00070
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375(19):1834‐1844. doi:10.1056/NEJMoa1607141
