11th January 2021, Dr Chee L Khoo

Sex hormones are not just responsible for the function of the reproductive system. They are also responsible for bone and cardiovascular health. Interestingly, they are produced, not only by the gonads, but also by other organs (1,2) including the central nervous system (CNS). Well, the eye is a neural structure and there is increasing evidence that oestrogens exert a neuro-protective role (3,4). Historically, interactions between gonadal hormones and the eye have received scarce attention. Recent research has begun to reveal possible links between oestrogens and eye diseases. This has implications on eye health when we use of anti-oestrogenic and anti-testosterone therapy.
Oestrogen receptors are divided into ERα and ERβ (5). ERα is predominantly expressed in reproductive tissues, the liver, and the central nervous system. Whereas ERβ is expressed in other tissues, including bone, endothelium, the lungs, the urogenital tract, the ovaries, the central nervous system, and the prostate (6). ERβ, may play a neuro-protective role in the retina through the inhibition of glutamate secretion.
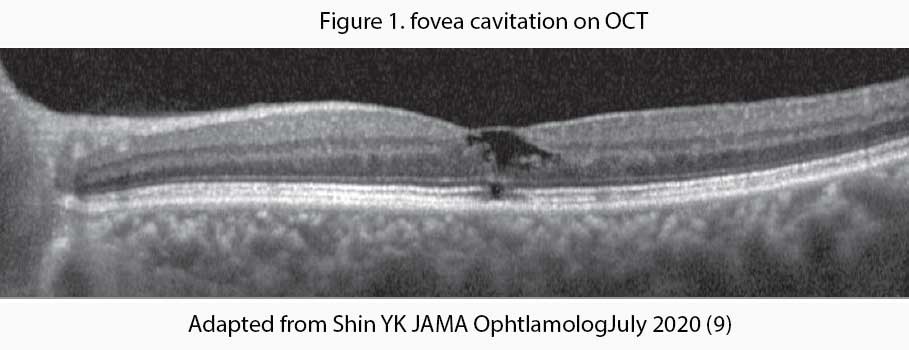
Tamoxifen, a selective estrogen receptor modulator, is associated with retinopathy and other macular abnormalities. Tamoxifen inhibits the glutamate-aspartate transporters that may result in Muller cell dysfunction and neuronal apoptosis which leads to the formation of cavitary spaces on optical coherence tomography (OCT) – see Figure 1. Fortunately, tamoxifen-associated macular abnormalities are rare and usually asymptomatic. Because it is usually asymptomatic, screening of patients receiving tamoxifen has become necessary for foveal cavitation even in the absence of crystals or visual complaints.
It is not just anti-oestrogenic agents that are implicated in eye diseases. Recently, studies (7,8) in male patients who received androgen deprivation therapy have reported the development of macular telangiectasia (MacTel) and age-related macular degeneration. We better have a look at what 5-ARI does and then how that relates to its potential effect on the eye.
5-alpha reductase converts testosterone to the more potent di-hydro-testosterone (DHT). A metabolite of DHT, 3β-androstanediol, is a potent and selective agonist of the oestrogen receptor ERβ and also binds to ERαwith a low affinity. Thus, 3β-androstanediol acts as an oestrogen which is important for eye health. 5-ARI inhibits the conversion of testosterone to DHT and hence, 5-ARIs may have anti-oestrogenic effects. The two common 5-ARIs that we know are finasteride (Proscar) which is commonly used for treatment of alopecia a duasteride (in Duodart) used in benign prostatic hypertrophy. 5-ARI treatment is used for transgender women as a component of gender-affirming hormone therapy.
Three types of isoenzymes were reported for 5α-reductase. Of the 3 isoforms of 5α-reductase, dutasteride inhibits all types (I, II, and III), whereas finasteride inhibits type II and III. In addition, dutasteride is 3 times more potent than finasteride in inhibiting type II and 100 times more potent in inhibiting type I. These results suggest that dutasteride is also more likely to have adverse effects.
A recent study assessed the association between the use of 5-alpha reductase inhibitors (5-ARI) and macular abnormalities on optical coherence tomography imaging (9). The study was a retrospective case-control, cross-sectional study. Electronic health record data of all male patients who visited Samsung Medical Centre from January 2016, to June 2019 were screened for foveal cavitation and inner retinal cyst on OCT imaging. The records were analysed for the history of 5-ARI use.
They identified 31 male patients who showed foveal cavitation on OCT imaging. 17 were excluded because they had well known cases of cystic change in the fovea. 9 patients had MacTel, 2 patients had retinal vein occlusion, 2 had vitreous or membrane traction, and 1 patient each had diabetic macular oedema, radiation retinopathy, myopic choroidal neovascularisation and retinoschisis. The remaining 14 patients had macular abnormalities of unknown cause.
The minimum duration of dutasteride treatment was 2.0 years, and the cumulative dose was 365.0 mg. The minimum duration of finasteride treatment was 2.5 years. and the cumulative dose was 5490.0 mg. 6 of 10 patients received dutasteride.
7 of 10 patients had hypertension, 2 had both hypertension and diabetes and 1 had diabetes. Similar to MacTel type 2, these findings suggest that the presence of systemic vascular disease may be a risk factor for 5-ARI associated with macular abnormalities.
The authors speculated that MacTel, tamoxifen-associated retinopathic findings and macular abnormalities associated with 5-ARIs may share the same developmental mechanism.
The study did not mentioned the number of records analysed. We don’t how prevalent these abnormalities are. Nonetheless, we do have large number of patients on 5-ARIs and manyn of these patients are on 5-ARIs indefinitely. Unfortunately, these abnormalities are largely asymptomatic and can only be diagnosed on OCT.
Should we be getting our patients who are in 5-ARIs, especially those who have systemic risk factors, to have their eyes checked by the ophthalmologist?
References:
- Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for a sex-specific medicines. Pharmacol Rev (2010) 62(2):155–98. doi:10.1124/pr.109.002071
- Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol (2003) 86(3–5):225–30. doi:10.1016/S0960-0760(03)00360-1
- Azcoitia I, Arevalo MA, De Nicola AF, Garcia-Segura LM. Neuroprotective actions of estradiol revisited. Trends Endocrinol Metab (2011) 22:467–73. doi:10.1016/j.tem.2011.08.002
- Melcangi RC, Panzica G, Garcia-Segura LM. Neuroactive steroids: focus on human brain. Neuroscience (2011) 191:1–5. doi:10.1016/j.neuroscience.2011.06.024
- Riggs BL, Hartmann LC. Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618-629. doi:10.1056/NEJMra022219
- Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential. Steroids. 2014;90:13-29. doi:10.1016/j.steroids.2014.06.012
- Muller S, Allam JP, Bunzek CG, Clemons TE, Holz FG, Charbel Issa P. Sex steroids and macular telangiectasia type 2. Retina. 2018;38(suppl 1):S61-S66. doi:10.1097/iae.0000000000001789
- Lin S-Y, Lin C-L, Chang C-H,Wu H-C, Lin C-H, Kao C-H. Risk of age-related macular degeneration in patients with prostate cancer: a nationwide, population-based cohort study. Ann Oncol. 2017;28 (10):2575-2580. doi:10.1093/annonc/mdx402
- Shin YK, Lee GW, Kang SW, Kim SJ, Kim AY. Macular Abnormalities Associated With 5α-Reductase Inhibitor. JAMA Ophthalmol. 2020 Jul 1;138(7):732-739. doi: 10.1001/jamaophthalmol.2020.1279. PMID: 32379286; PMCID: PMC7206535.
- Nuzzi R, Scalabrin S, Becco A, Panzica G. Gonadal Hormones and Retinal Disorders: A Review. Front Endocrinol (Lausanne). 2018 Mar 2;9:66. doi: 10.3389/fendo.2018.00066. PMID: 29551993; PMCID: PMC5840201.
