25th April 2021, Dr Chee L Khoo

Chronic rhinosinusitis is a very common problem in general practice. Despite the “-itis”, infection is not actually the core problem. That is why antibiotic scripts after antibiotics script isn’t always the solution. There is often a significant inflammatory/allergic component in the pathogenesis. Current standard of care consists of intranasal steroids, nasal saline irrigation and short courses of systemic corticosteroids. For the more severe cases, surgical drainage procedures are necessary (eg. antrostomies, polypectomies, correction of nasal septal deviation, turbinectomies, etc). However, surgery is rarely definitive as the postoperative recurrence rates are high. Many patients often end up having surgery every 5-8 years. What else can we do?
In several allergic diseases including allergic rhinitis and asthma, eosinophils have an important role in the pathogenesis. In these conditions, there are large numbers of eosinophils in circulation, ariways and sputum. Interleukin-5 (IL5) is a key mediator of eosinophils activation. Drugs that target IL-5 are mepolizumab and reslizumab.
Mepolizumab is a humanised monoclonal antibody that selectively binds to and inactivates IL-5, thereby inhibiting eosinophilic inflammation (1). Mepolizumab have been shown to reduce eosinophilic inflammation in severe eosinophilic asthma, eosinophilic granulomatosis with polyangiitis and hyper-eosinophilic syndrome.
What about mepolizumab in rhinosinusitis? Previous studies have shown reductions in polyp size, improved symptoms, and a reduced need for nasal surgery in patients with chronic rhinosinusitis with nasal polyps receiving 750 mg of intravenous mepolizumab (2,3).
SYNAPSE is a randomised, double-blind, placebo-controlled, parallel-group, phase 3 trial done at 93 centres in 11 countries designed to assess the efficacy and safety of mepolizumab at a dose of 100 mg administered subcutaneously 4 weekly for 52 weeks, compared with placebo. Patients have recurrent, refractory, severe, bilateral chronic rhinosinusitis with nasal polyps who were eligible for repeat surgery. Patients had to have at least one nasal surgery of the paranasal sinuses and removal of polyp tissue from the nasal cavity and the sinuses in the past 10 years.
The explored the following outcomes:
- Nasal obstruction (VAS score)
- Total endoscopic nasal polyp score
- Time-to-first nasal surgery
- Proportion of patients requiring systemic corticosteroids for nasal polyps
What did they find?
From May, 2017 to Dec, 2018, 414 patients were randomly assigned with 407 included in the intention to treat population – 206 received mepolizumab and 201 received placebo. 15 patients (8 from mepolizumab and 7 from placebo group) discontinued treatment and withdrew from the study. A further 42 patients (15 receiving mepolizumab and 27 placebo) discontinued treatment and continued being assessed.
Patient demographics and baseline characteristics were similar between treatment groups. A lower proportion of patients in the mepolizumab versus placebo group had two or more previous nasal surgeries and aspirin-exacerbated respiratory disease and patients in the mepolizumab group had lower total endoscopic nasal polyps scores at baseline.
Compared with placebo, add-on mepolizumab to standard care reduced polyp size, nasal obstruction symptoms, reduced the occurrence of nasal surgery and reduced the need for systemic corticosteroids. See Table 1.
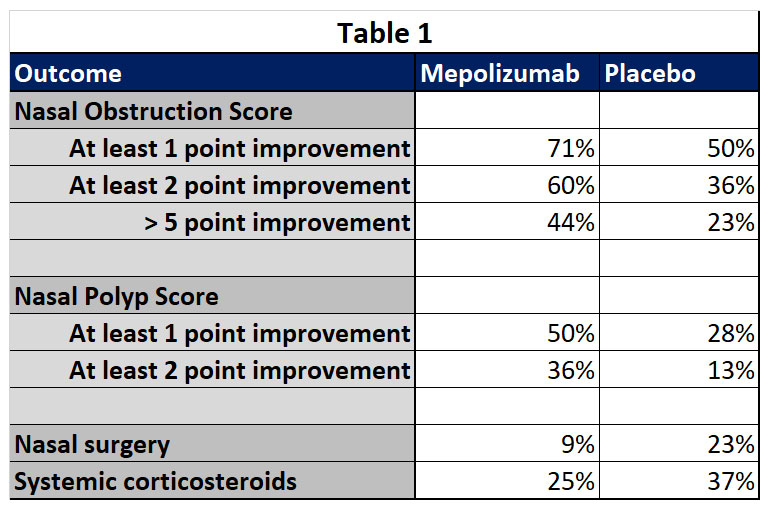
Treatment effects were seen at 4 weeks after the first dose and were sustained until week 52. Remember, these are patients who had one or more nasal surgery before study entry, their chronic rhinosinusitis with nasal polyps was considered refractory to medical and surgical treatment.
There has been success in treating refractory rhinosinusitis with other monoclonal antibodies. Dupilumab, an anti-IL-4 receptor antibody, and omalizumab, an anti-IgE antibody, are also efficacious in patients with chronic rhinosinusitis with nasal polyps, as shown in the SINUS (5) and POLYP (6) studies.
Symptoms of chronic severe rhinosinusitis include nasal blockage, loss of smell, facial pressure, and rhinorrhoea can have a substantial impact on health-related quality of life. Nasal surgery is not without consequences. Multiple endoscopic nasal surgeries can reduce sense of smell (7,8). We can expect monoclonal antibodies as standard care for refractory rhinosinusitis flooding the market in the coming years.
References:
- Flood-Page P, Menzies-Gow A, Phipps S, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest 2003; 112: 1029–36
- Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol 2011; 128: 989–95.
- Bachert C, Sousa AR, Lund VJ, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: randomized trial. J Allergy Clin Immunol 2017; 140: 1024–31.e14
- Han JK, Bachert C, Fokkens W, Desrosiers M, Wagenmann M, Lee SE, Smith SG, Martin N, Mayer B, Yancey SW, Sousa AR, Chan R, Hopkins C; SYNAPSE study investigators. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021 Apr 16:S2213-2600(21)00097-7. doi: 10.1016/S2213-2600(21)00097-7. Epub ahead of print. PMID: 33872587.
- Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019; 394: 1638–50.
- Gevaert P, Omachi TA, Corren J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol 2020; 146: 595–605
- Nguyen DT, Bey A, Arous F, Nguyen-Thi PL, Felix-Ravelo M, Jankowski R. Can surgeons predict the olfactory outcomes after endoscopic surgery for nasal polyposis? Laryngoscope 2015; 125: 1535–40.
- Seys SF, De Bont S, Fokkens WJ, et al. Real-life assessment of chronic rhinosinusitis patients using mobile technology: the mySinusitisCoach project by EUFOREA. Allergy 2020; 75: 2867–78.
