1st January 2022, Dr Chee L Khoo
This definitely wasn’t in the curriculum when I left med school (alright, it was more than 30 years ago now) but I don’t remember being updated about its relevance. I am talking about the epidermal growth factor receptor (EGFR). It is not really in the realm of general practice as such but we do have patients coming back from the oncologist with a tumour bearing an EGFR gene mutation. Because EGFR has been found to be over-expressed in many tumours of epithelial origin, it is a potential target for antitumor therapy. We ought to know the significance of EGFR to the patient sitting in front of us.
What is epidermal growth factor (EGF)?
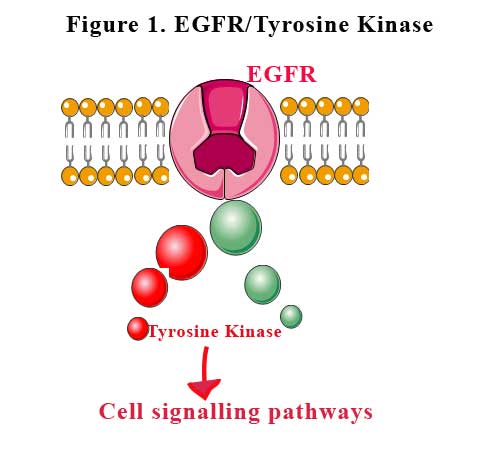
Epidermal growth factor receptor is a transmembrane protein which is a receptor for members of the epidermal growth factor family of extracellular protein ligands. Binding of the ligand to the receptor allows the receptor to attach to another nearby epidermal growth factor receptor protein (dimerise) and activating the receptor complex. The activated receptors bind to intracellular signalling proteins and stimulate the activation of many signaling pathways that promote cell growth, cell proliferation and cell survival. See Figure 1.
EGFR is one of 4 ErbB family of receptors which are structurally related to receptor tyrosine kinases. EGFR is the most extensively studied of the ErbB receptors. A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an “on” or “off” switch in many cellular functions.
In humans, the ErbB family members are:
Activation can occur with ligand-dependent in heterodimers with EGFR, HER3 and HER4 or with ligand-independent dimerisation in the case of HER2 homodimers.
The gene symbol, ErbB, is derived from the name of a viral oncogene to which these receptors are homologous: erythroblastic leukemia viral oncogene.
What can go wrong with EGFR?
Protein kinases can become mutated, stuck in the “on” position, and cause unregulated growth of the cell, which is a necessary step for the development of cancer. Therefore, tyrosine kinase inhibitors are effective cancer treatments.
Insufficient ErbB signaling in humans is associated with the development of neurodegenerative diseases, such as multiple sclerosis and Alzheimer’s disease while overexpressed or mutated EGFR are present in many cancers, especially in breast cancer, ovarian cancer, and non-small cell lung cancer (2). The overexpression and overactivation of ErbB receptors are correlated with poor prognosis, drug resistance, cancer metastasis, and lower survival rate. ErbB receptors, especially EGFR and ErbB2 have been the primary choices as targets for developing cancer therapies.
EGFR and lung cancer
Approximately 85% of lung cancers are classified as non–small cell lung cancer (NSCLC), and include lung adenocarcinoma, squamous cell carcinoma (SCC), and large cell carcinoma (LCC) histologic subtypes. Mutations in the tyrosine kinase domain of EGFR are present in about 15% of Caucasian and nearly 50% of Asian patients with advanced NSCLC (2,3). Almost 90% of these mutations consist of deletions in exon 19 or L858R point mutations within exon 21. These genetic changes act as oncogenic drivers leading to ligand-independent activation of EGFR downstream signalling, thus promoting cell proliferation, survival and migration. The identification of these mutations in patients with NSCLC and the subsequent development of targeted therapy with small-molecule EGFR tyrosine kinase inhibitors (TKIs) has dramatically revolutionised the treatment landscape of these tumours.
Large-scale Phase 2/3 clinical trials have consistently demonstrated the superior efficacy of first-generation (gefitinib and erlotinib) and second-generation (afatinib, dacomitinib, neratinib, canertinib) TKIs in comparison with standard first-line platinum-based chemotherapy for the treatment of patients with advanced NSCLC with activating EGFR mutations (4). The objective response rate (ORR) to these first and second generation EGFR-TKIs is remarkably high (60–70%) but the majority of patients develop resistance, with average progression-free survival (PFS) ranging from 9 to 15 months (4).
Newer, third generation EGFR-TKIs with specific capability bind to T790M mutated receptor have been developed and successfully tested in patients with acquired resistance [5,6]. The most famous of the third generation TKI is osimertinib. Osimertinib is approved worldwide for the treatment of patients with metastatic NSCLC with EGFR T790M mutations that show disease progression after responding to first or second generation EGFR-TKIs. From a number of clinical trials, median time on sequential afatinib and osimertinib treatment was 27.7 months for this patient population with EGFR mutations. More favourable outcomes were seen in patients with Del19-positive disease and Asian patients, with prolonged median time on treatment and a median overall survival (OS) of over 3.5 years reported for both subgroups.
Osimertinib is also approved for first-line treatment of patients with metastatic NSCLC harbouring the specific EGFR mutation exon 19 deletion or exon 21 Leu858Arg mutation (L858R).
However, patients with a major mutation on the C797S site on the EGFR gene showed resistance to osimertinib. Chemotherapy is often the only option for patients who progress on osimertinib treatment in everyday clinical practice. Fourth-generation EGFR-TKIs have been developed in order to successfully target the C797S mutant EGFR and other EGFR mutations.
EGFR and breast cancer
Triple-negative breast cancer (TNBC) is characterised by the lack of oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2). EGFR is, besides HER3, the preferred heterodimerisation partner of HER2 (7), but its role in HER2-overexpressing cancer is still not fully understood [8,9].
EGFR overexpression in breast cancer is associated with large tumour size, poor differentiation, and poor clinical outcomes. Though EGFR overexpression is observed in all subtypes of breast cancer, EGFR is more frequently overexpressed in triple-negative breast cancer (TNBC) and inflammatory breast cancer (IBC), which is especially aggressive. Treatment of patients with these phenotypes has been challenging not only because of the aggressive behaviour of these diseases but also because of the lack of established clinically relevant treatment targets. The role of EGFR in breast cancer has been scrutinised, and several therapies that target EGFR have been developed.
Cetuximab is a chimeric anti-EGFR monoclonal antibody. Cetuximab has a synergistic effect with radiotherapy or chemotherapy and can be used in the treatment of triple-negative breast cancer cells (TNBCs) that are overexpressed EGFR. Gefitinib is a small molecule drug that irreversibly inhibits EGFR receptor (tyrosine kinase inhibitor). Vandetanib is an oral active antagonist of EGFR (ErbB1or HER1), vascular endothelial growth factor receptor-2 (VEGFR-2) and RET kinase. Erlotinib is an orally potent EGFR inhibitor. It is used for the treatment of pancreatic cancer and non-small cell lung cancer. However, it showed less activity in metastatic breast cancer (MBC) women therapy. Furthermore, combination of erlotinib with docetaxel/capecitabine can be used in MBC treatment.
There are multiple receptor blockers as well. Neratinib is an irreversible pan-tyrosine kinase inhibitor that also demonstrates the activity against HER1, HER2, and HER4. Afatinib is an oral, small molecule anilino-quinazoline compound which is a highly selective inhibitor of EGFR/HER1, HER2, and HER4 tyrosine kinase activity. This drug can be used alone or in combination with other treatment in HER2-positive breast cancer (10).
So, you can see that EGFR and similar receptors feature quite prominently in many cancers especially in lung and breast cancers. We can be feel very optimistic with our patients when they are informed that they have a EGFR mutation in their cancers.
References:
- https://en.wikipedia.org/wiki/Epidermal_growth_factor_receptor. Accessed 31st December 2021.
- Rosell, R., Moran, T., Queralt, C., Porta, R., Cardenal, F., Camps, C. et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med 361, 958–967 (2009).
- Shi, Y., Au, J. S.-K., Thongprasert, S., Srinivasan, S., Tsai, C.-M., Khoa, M. T. et al. A Prospective, Molecular Epidemiology Study of EGFR Mutations in Asian Patients with Advanced Non–Small-Cell Lung Cancer of Adenocarcinoma Histology (PIONEER). J. Thorac. Oncol. 9, 154–162 (2014).
- Lim, S. M., Syn, N. L., Cho, B. C. & Soo, R. A. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Treat. Rev. 65, 1–10 (2018).
- Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, Haggstrom D, Felip E, Kim JH, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. The New England journal of medicine. 2015; 372:1689–1699.
- Kim ES. Olmutinib: First Global Approval. Drugs. 2016; 76:1153–1157.
- Lemmon, M.A.; Schlessinger, J.; Ferguson, K.M. The EGFR family: Not so prototypical receptor tyrosine kinases. Cold Spring Harb. Perspect. Biol. 2014, 6, a020768.
- Guo, P.; Pu, T.J.; Chen, S.A.; Qiu, Y.; Zhong, X.R.; Zheng, H.; Chen, L.; Bu, H.; Ye, F. Breast cancers with EGFR and HER2 co-amplification favor distant metastasis and poor clinical outcome. Oncol. Lett. 2017, 14, 6562–6570
- Ingthorsson, S.; Andersen, K.; Hilmarsdottir, B.; Maelandsmo, G.M.; Magnusson, M.K.; Gudjonsson, T. HER2 induced EMT and tumorigenicity in breast epithelial progenitor cells is inhibited by coexpression of EGFR. Oncogene 2016, 35, 4244–4255
- Al-Mahmood S, Sapiezynski J, Garbuzenko OB, Minko T. Metastatic and triple-negative breast cancer: challenges and treatment options. Drug Deliv Transl Res. 2018 Oct;8(5):1483-1507. doi: 10.1007/s13346-018-0551-3. PMID: 29978332; PMCID: PMC6133085.
