12th April 2022, Dr Chee L Khoo
It’s almost close to a wonder drug. It is widely used in type 2 diabetes (T2D) for management of hyperglycaemia when the HbA1c is >7.0%. For that indication, it is pretty potent and in clinical trials, can reduce the HbA1c by 1-1.5%. It also assists in weight reduction and thence, reduce the core problem of insulin resistance in these patients. In put cream on top, it also have significant cardiovascular and renal benefits. It is also used “off-label” as an anti-obesity drug in patients without diabetes. Of course, we are referring to the GLP1-receptor agonists (GLP1-RA). There is no free lunch here. Several randomised control trials have shown a higher rate of gallbladder and biliary disorders in patients using these agents compared with placebo. How significant is this adverse signal?
In the STEP 3 randomised trial, once weekly semaglutide 2.4 mg used in obese adults without diabetes was associated with 4.9% incidence of gallbladder related disorders (mainly cholelithiasis) compared with 1.5% in the placebo group (1). In another randomised controlled trial, there was an increased incidence of cholelithiasis in patients receiving once daily liraglutide 3mg compared with placebo (0.45% vs 0%) during the 12 months (2).
The LEADER trial was an international, randomised, double-blind, controlled cardiovascular outcomes trial (CVOT). Participants with T2D at high risk for cardiovascular (CV) events (n = 9,340) were randomised 1:1 to receive either liraglutide (≤1.8 mg daily; n = 4,668) or placebo (n = 4,672), with both groups also receiving standard care (treatment period: 3.5–5 years). Acute gallstone disease was a medical event of special interest. A post hoc analysis categorised adverse events of acute gallbladder or biliary disease into four groups: uncomplicated gallbladder stones, complicated gallbladder stones, cholecystitis and biliary obstruction (3).
There was an increased risk of acute gallbladder or biliary disease with liraglutide versus placebo (n = 141 of 4,668 vs. n = 88 of 4,672 patients, respectively. While the hazard ratio (HR) was 1.60, the absolute numbers were small (3% vs 0.5%). Similar trends were observed for each of the four categories of gallbladder- or biliary tract–related events. Cholecystectomy was performed more frequently in liraglutide-treated patients (HR 1.56) but for similar proportions of the patients who experienced gallbladder- or biliary tract–related events (57% with liraglutide vs. 59% with placebo).
GLP-1 RAs are generally prescribed at higher doses for weight loss rather than for control of T2D, do GLP1-RA therapy in patients with T2D have the same incidence of gallbladder or biliary diseases compared with GLP1-RA in patients treated for obesity? In complicate things further, patients with T2D do have a higher risk of gallbladder or biliary diseases anyway.
Because of the gap in our knowledge and the small absolute numbers seen in the trials, a systematic review and meta-analysis was conducted to determine if risks differ by indication (for diabetes vs weight loss), dose, or duration of treatment (4). The authors searched for randomised clinicals trials of GLP-1 RA medications (albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, or semaglutide) that also reported adverse events of gallbladder or biliary diseases. The literature search was conducted of MEDLINE, Cochrane Library, EMBASE, and Web of Science from inception to June 30, 2021,with no language restrictions.
The primary outcome was a composite of gallbladder or biliary diseases, including gallbladder disorders and biliary related events. Secondary outcomes were 3 subcategories of gallbladder and biliary diseases, including bile duct obstruction, stenosis, and stone; biliary colic, cyst, and fistula; biliary tract cancer; cholecystectomy, cholecystitis, cholelithiasis and cholangitis.
They evaluated the associations with gallbladder or biliary diseases of specific GLP-1 RA medications, higher and lower doses, shorter and longer treatment durations (≤26 or >26weeks), indication for treatment (type 2 diabetes/other diseases or obesity), type of control (placebo or active comparator), and baseline BMI.
What did they find?
76 RCTs with 77 data sets were included in the meta-analysis. The included studies had a combined total of 103 371 participants (mean[SD]age,57.8[6.2] years; 41 868 [40.5%] women; mean BMI, 32.6; mean HbA1c, 7.8%). 91,599 (84.4%) of the trial participants had T2D.
Overall effects
GLP1-RA treatment is associated with a 37% increase in gallbladder or biliary diseases compared with placebo. The absolute risk difference was an additional 27 (range 17-38) events per 100,000 patients per year.
Are all the GLP1-RAs the same?
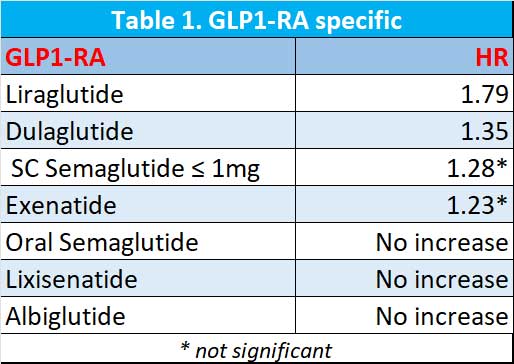
The worse of the GLP1-RA appears to be liraglutide. Dulaglutide appears be better than liraglutide while low dose weekly semaglutide (≤ 1mg weekly) and exenatide non-significantly increase the risk of gallbladder or biliary diseases. Oral semaglutide, lixisenatide and albiglutide did not increase the risk. See Table 1.
Dose, duration, indication of treatment
Use of GLP-1 RAs was significantly associated with increased risks of gallbladder or biliary disease at higher doses (RR, 1.56; 95%CI, 1.36-1.78) but not at lower doses (RR,0.99;95%CI,0.74-1.33; P = .006 for interaction). Longer duration of treatment with GLP-1 RAs (>26 weeks) was associated with increased risk for gallbladder or biliary disease (RR, 1.40; 95% CI, 1.26-1.56), but shorter duration (=26 weeks) of treatment was not (RR 0.79). The effects of dose and duration were similar in trials for diabetes.
Use of GLP-1Ras for weight loss showed stronger effects on the risk of gallbladder or biliary disease than the other indications.
In summary, to treatment with GLP1-RAs compared with placebo or active controls was associated with increased risk of the composite outcome of gallbladder or biliary diseases and for cholelithiasis, cholecystitis, and biliary diseases. Risk was increased in trials of patients treated for diabetes and for weight loss and was higher in the trials for weight reduction. Higher doses and longer duration of GLP-1 RAs treatment were also associated with increased risk of gallbladder or biliary diseases, although the association was not statistically significant.
Of note though, the absolute numbers are small. Higher doses of the GLP1-RA are not yet available in Australia under the TGA but will be soon.
References:
- Wadden TA, Bailey TS, Billings LK, et al; STEP 3 Investigators. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403-1413. doi:10.1001/jama.2021.1831
- Lundgren JR, Janus C, Jensen SBK, et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. N Engl J Med. 2021;384(18): 1719-1730. doi:10.1056/NEJMoa2028198
- Nauck MA, Muus Ghorbani ML, Kreiner E, Saevereid HA, Buse JB; LEADER Publication Committee on behalf of the LEADER Trial Investigators. Effects of liraglutide compared with placebo on events of acute gallbladder or biliary disease in patients with type 2 diabetes at high risk for cardiovascular events in the LEADER randomized trial. Diabetes Care. 2019;42(10):1912-1920. doi:10.2337/dc19-0415
- He L, Wang J, Ping F, Yang N, Huang J, Li Y, Xu L, Li W, Zhang H. Association of Glucagon-Like Peptide-1 Receptor Agonist Use With Risk of Gallbladder and Biliary Diseases: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Intern Med. 2022 Mar 28. doi: 10.1001/jamainternmed.2022.0338. Epub ahead of print. PMID: 35344001.
