12th July 2023, Dr Chee L Khoo
The WHO define biological markers or biomarkers as “any substance, structure or process that can be measured in the body or its products and influence or predict the incidence of outcome or disease”. In heart failure, many biomarkers have been studied but B-type natriuretic peptide (BNP) and N-terminal pro–B-type natriuretic peptide (NT-proBNP) come close to the characteristics of “ideal” heart failure (HF) biomarkers. While the measurement of either of these natriuretic peptides (NP) are not funded under Medicare, they are often used in clinical practice. Like all biomarkers, we need to understand the sensitivity and specificity of these markers and their roles in different settings of HF.
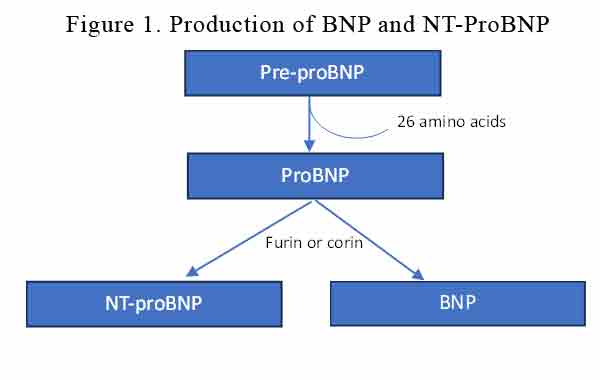
BNP is produced primarily by ventricular cardiomyocytes in response to volume or pressure overload. BNP and NT-proBNP are synthesised from a pre-hormone (pre-proBNP) of 134 amino acids, encoded by the NPPB gene. 26 aminoacids are cleaved off the preproBNP to become proBNP which is converted by furin or corin into BNP and NT-proBNP. See Figure 1.
Circulating BNP and NT-proBNP levels are normally very low but increase significantly in HF patients as a mechanism to restore normal haemodynamics. BNP promotes arterial vasodilation, diuresis, and natriuresis, exerts anti-hypertrophic and anti-fibrotic effects, and counteracts the activation of RAAS, SNS and the endothelin systems. 25% of BNP is excreted by the kidneys while the rest is metabolised by neprilisyn. NT-proBNP is fully excreted by the kidneys. NT-proBNP has a longer half life (3X) and higher (6X) plasma levels than BNP.
How can we use BNP and NT-proBNP?
Biomarkers can serve either as “antecedent biomarkers” (predicting future disease development), screening biomarkers, diagnostic biomarkers, staging biomarkers, prognostic and therapeutic biomarkers as well as inclusion/exclusion and outcome criteria for clinical trials.
Diagnosis (or exclusion)
Any condition which causes a strain to the cardiac ventricles will increase NP. In 1586 patients with new onset dyspnoea admitted to Emergency Department, BNP levels were significantly higher in subjects with acute HF, and plasma BNP increased in parallel with the New York Heart Association (NYHA) class (2). When the cut off level of 100 ng/L is used, they had 90% sensitivity, 76% specificity and 83% accuracy.
In the PRIDE study, in 600 patients admitted to the emergency department (ED) for dyspnoea, NT-proBNP < 300 ng/L ruled out effectively acute HF (99% negative predictive value [NPV]), and rule-in cutoffs of 450 ng/L in subjects with < 50 years (93% sensitivity, 95% specificity, 95% accuracy) and 900 ng/L in subjects with ≥ 50 years (91% sensitivity, 80% specificity, 85% accuracy) were proposed [3].
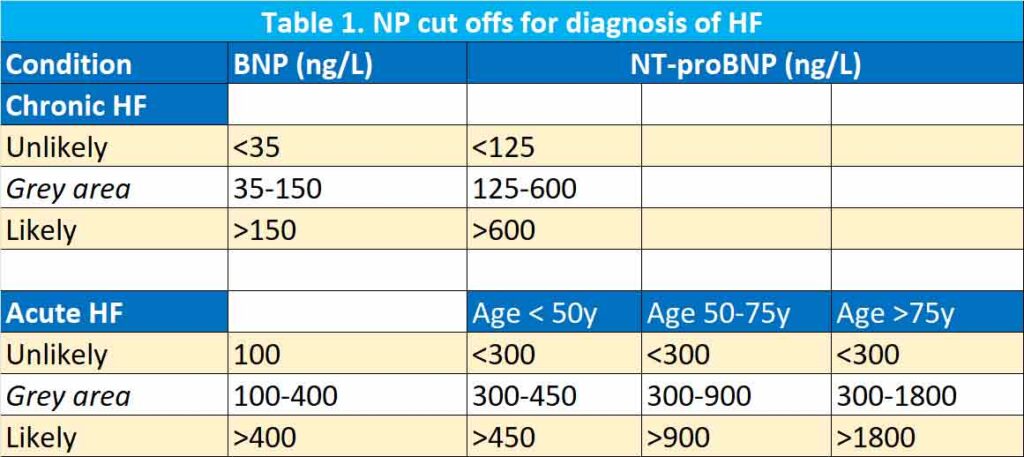
NP levels, in general, increase with age probably related to the reduction in left ventricular compliance and renal function with ageing. Further, there is a difference whether the HF is acute or chronic and whether the presentation is in the community or at ED. Thus, European Society of Cardiology (ESC) guidelines recommend the use of BNP and NT-proBNP for the exclusion of HF with reference values < 100 ng/L and < 300 ng/L for acute HF, respectively, and < 35 ng/L and < 125 ng/L for chronic HF, respectively (1). See Table 1.
Risk stratification tool
Not only is NP levels useful in diagnosing (or excluding) HF, it is a good risk stratification tool as well. In the ADHERE trial, in 48,629 patients with acute HF, admission BNP levels were associated with intra-hospital mortality (4). A systematic review of 19 studies revealed that the relative risk of all-cause death increases by 35% for each 100 ng/L increase in admission BNP (5). Similarly, NT-proBNP can predict the short- and long-term prognosis in patients with acute HF [6, 7].
Interestingly, discharge BNP levels were even more predictive of 1 year mortality than admission BNP. The OPTIMIZE trial demonstrated that discharge BNP was more predictive of 1-year death (hazard ratio [HR] 1.34 and 1-year death or hospitalization (HR 1.15) than admission BNP [5].
The change between admission and discharge BNP was also useful in risk prediction. In hospitalised patients whose NT-proBNP reduced by <50% were 57% more likely to die or re hospitalised at 12 months compared with those whose NT-proBNP levels reduced by >50% (8).
Similar findings were observed with both BNP and NT-proBNP levels in patients with chronic heart failure.
Screening
It would be most useful if we can predict who may develop HF. Well, NP can also help here. In an analysis of 3346 subjects without clinical evidence of HF followed for an average of 5 years, baseline BNP or NT-proBNP > 80th percentile were associated with a significantly higher risk of new-onset HF (BNP: HR 3.07, p = 0.002; NT-proBNP: HR 5.02, p < 0.001) [9].
in a sub-analysis of the Prevention of Events With Angiotensin-Converting Enzyme Inhibition (PEACE) study on 3761 subjects with stable coronary artery disease and preserved LVEF, BNP, and NT-proBNP were strong predictors of HF development during a median 5-year follow-up [10].
Just like in risk stratification, changes in NP are also useful in better predicting new onset HF. Patients whose NP increased by >25% between measurements are ~2X more likely to developed HF (11).
In a novel study, STOP-HF, patients with risk factors for HF but no clinical signs of HF were referred for echocardiography whenever BNP was >50ng/L. Intensive lifestyle changes and RAAS therapy over a 4 year period demonstrated a reduction in LV dysfunction (12).
The ACC/AHA guidelines suggest that NPs should be used to screen subjects at risk of developing HF to optimise medical therapy and prevent LV dysfunction.
Guide to therapy
Here at GPVoice, we have emphasised many times how important that we need to follow guideline-directed medical therapy (GDMT) for patients with HF. Patients with HF have high mortality rates and each time they are hospitalised with exacerbations of their HF, LV dysfunction increases. Response to GDMT is gauged by symptoms and echocardiographic improvement in EF.
Changes in plasma NPs may be used as surrogate end-points to assess the efficacy of new HF therapies. There were a number of studies looking at using a titration strategy guided by BNP or NT-proBNP vs standard care in patients with chronic HF. Initial results suggested that a NP-guided treatment is associated with lower rates of all-cause mortality and HF hospitalisation [13-15].
However, in the GUIDE-IT trial, NT-proBNP-driven management did not reduce healthcare costs nor improved the quality of life [16]. The study was discontinued for futility after the enrolment of 894 patients, with a median follow-up of 15 months, because of no difference in the primary endpoint (cardiovascular death or HF hospitalization: HR 0.98, p = 0.88). None of the secondary endpoints (individual components of the primary endpoint, all-cause death, HF hospitalisations, days to hospitalization for cardiovascular causes, adverse events) differed significantly between the two groups [17].
In summary, the diagnosis of HF is not always straight forward. We can’t depend on the absence of symptoms in excluding the presence of HF. Often but not always, echocardiography will confirm or exclude the diagnosis but even cardiologists sometimes are unsure and have to rely on biomarkers.
BNP and NT-proBNP have been intensively studied and has a role in diagnosis, risk stratification, screening and perhaps, one day, guide our therapy in patients with HF.
References:
- Castiglione, V., Aimo, A., Vergaro, G. et al. Biomarkers for the diagnosis and management of heart failure. Heart Fail Rev 27, 625–643 (2022).
- Maisel AS, Krishnaswamy P, Nowak RM et al (2002) Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 347:161–167.
- Januzzi JL, Camargo CA, Anwaruddin S et al (2005) The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol 95:948–954.
- Fonarow GC, Peacock WF, Phillips CO et al (2007) Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol 49:1943–1950.
- Kociol RD, Horton JR, Fonarow GC et al (2011) Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail 4:628–636.
- Januzzi JL, van Kimmenade R, Lainchbury J et al (2005) NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: The International Collaborative of NT-proBNP Study. Eur Heart J 27:330–337.
- Januzzi JL, Sakhuja R, O’Donoghue M et al (2006) Utility of amino-terminal pro-brain natriuretic peptide testing for prediction of 1-year mortality in patients with dyspnea treated in the emergency department. Arch Intern Med 166:315–320.
- Michtalik HJ, Yeh H-C, Campbell CY et al (2011) Acute changes in N-terminal pro-B-type natriuretic peptide during hospitalization and risk of readmission and mortality in patients with heart failure. Am J Cardiol 107:1191–1195.
- Wang TJ, Larson MG, Levy D et al (2004) Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 350:655–663.
- Omland T, Sabatine MS, Jablonski KA et al (2007) Prognostic value of B-type natriuretic peptides in patients with stable coronary artery disease: the PEACE Trial. J Am Coll Cardiol 50:205–214.
- deFilippi CR, Christenson RH, Gottdiener JS et al (2010) Dynamic cardiovascular risk assessment in the elderly: the role of repeated amino terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol 55:441–450
- Ledwidge M, Gallagher J, Conlon C et al (2013) Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 310:66–74.
- Troughton RW, Frampton CM, Brunner-La Rocca HP et al (2014) Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J 35:1559–1567.
- Felker GM, Hasselblad V, Hernandez AF, O’Connor CM (2009) Biomarker-guided therapy in chronic heart failure: a meta-analysis of randomized controlled trials. Am Heart J 158:422–430.
- Savarese G, Trimarco B, Dellegrottaglie S et al (2013) Natriuretic peptide-guided therapy in chronic heart failure: a meta-analysis of 2,686 patients in 12 randomized trials. PLoS ONE 8:e58287.
- Mark DB, Cowper PA, Anstrom KJ et al (2018) Economic and quality-of-life outcomes of natriuretic peptide-guided therapy for heart failure. J Am Coll Cardiol 72:2551–2562.
- Felker GM, Anstrom KJ, Adams KF et al (2017) Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 318:713–720.
