13th July 2023, NIA Diagnostic Imaging
Aortic aneurysms are known as silent killers as the majority of patients with the condition are asymptomatic and are usually found incidentally during physical examinations or diagnostic imaging studies performed for other reasons (Faiza & Sharman, 2023). Ruptured aortic aneurysms are associated with high mortality rates of approximately 80-90% for patients with ruptured AAAs (Wise et al., 2016) in which “more than 50% of patients die before they reach the emergency room” (Shaw et al., 2023). Imaging plays an imperative role in aortic aneurysm diagnosis, monitoring of its growth and risk of rupture, preoperative evaluation and planning of repair, treatment procedures, post-operative follow-up and lifelong surveillance (Robinson et al., 2013).
Risk factors for aortic aneurysms can include the following: advanced age (over 50 years old), male sex, smoking, chronic obstructive pulmonary disease, hypercholesterolaemia, hypertension, family history of aortic aneurysms and history of cardiovascular diseases (Anagnostakos & Lal, 2021; Bertino & Mustafaraj, 2017).
Aortic aneurysms are often incidental findings and confirmed by ultrasound (US) or computed tomography (CT) imaging. Further imaging and intervention will be dependent on the size of the aneurysm. Early detection of aortic aneurysms allows for monitoring of aneurysm growth, appropriate intervention of modifiable risk factors (e.g. cessation of smoking and cardiovascular health management through antiplatelet therapy, statin therapy or anti hypertensive therapy) and prompt treatment to repair and prevent aneurysm rupture (Robinson et al., 2013).
Screening studies have demonstrated improvement in treatment and cardiovascular health outcomes in male patients on surveillance and reduction in risk of AAA-related mortality by approximately 50% in men aged 65 to 74 years (Aggarwal et al., 2011; Theivendran & Chuen, 2018).
US is considered the imaging modality of choice for screening and monitoring aortic aneurysms due to its wide availability, non-invasive nature, non-use of ionising radiation and reliability in detecting and measuring aortic aneurysms (Aggarwal et al., 2011). CT Angiography (CTA) is primarily the first-line imaging modality for pre-operative and post-operative evaluation of aortic aneurysms (Pandey & Litt, 2015). The use of intravenous contrast administration, multi-phase imaging, multiplanar analysis and 3D reconstruction capabilities of CTA allows for extensive assessment of aneurysm location and morphology (e.g. size, shape, tortuosity and presence of calcification and mural thrombus) as well as its involvement with other branch vessels and structures to evaluate patient candidacy for aneurysm repair via endovascular or open surgery (Pandey & Litt, 2015). Due to its high spatial resolution and inter-reader reproducibility, CTA is often used for both serial post-operative assessment and post endovascular aneurysm repair (EVAR) surveillance (Daye & Walker, 2018).
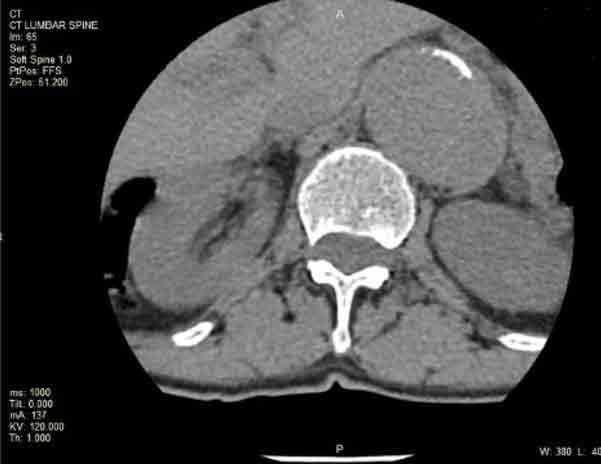
A 75-year-old female patient presented for a CT lumbar spine for lower back pain which showed incidental findings of aneurysmal dilation of the descending aorta and abdominal aorta. A CT aortogram was suggested to further evaluate the size, extent and location of the aortic aneurysms. Findings from the scan demonstrated aneurysmal dilatation of the ascending aorta, aortic arch and descending aorta measuring up to 5 cm in maximal axial dimension.

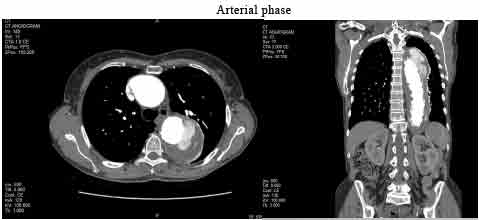
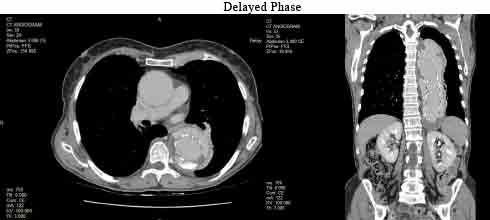
A CT aortogram with a three-phase scan protocol (non-contrast, arterial and delayed) was performed a few months later following recent endograft repair of an aneurysm of the descending aorta. The CT scan findings indicate extravasation/leakage of contrast outside the graft at the proximal and distal attachments seen on arterial and delayed imaging, which is suggestive of a type 1A/1B endoleak. This type of endoleak results from inadequate sealing of the proximal and distal ends of the endoluminal stent and requires urgent treatment due to the high aneurysm sac pressure and risk of rupture (Kassem, 2017). In this case, it is evident that medical imaging plays a critical role in the early detection, intervention and surveillance of aortic aneurysms and therefore improving patient outcomes and quality of life.
With the benefits of having skilled sonographers, radiographers and radiologists on site, both diagnostic ultrasound and CT services are readily accessible at all NIA Diagnostic Imaging clinics, where urgent same-day appointments and reports are guaranteed. You are invited to visit www.niaimaging.com.au for detailed information.
References:
- Aggarwal, S., Qamar, A., Sharma, V., & Sharma, A. (2011). Abdominal aortic aneurysm: A comprehensive review. Experimental and clinical cardiology, 16(1), 11–15. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3076160/
- Anagnostakos, J., & Lal, B. K. (2021). Abdominal aortic aneurysms. Progress in Cardiovascular Diseases, 65, 35-43. https://doi.org/10.1016/j.pcad.2021.03.009
- Badger, S., Forster, R., Blair, P. H., Ellis, P., Kee, F., & Harkin, D. W. (2017). Endovascular treatment for ruptured abdominal aortic aneurysm. Cochrane Database of Systematic Reviews, 5(5), 1-39 doi: 10.1002/14651858.CD005261.pub4
- Bertino, R. E. & Mustafaraj, E. (2017). The Retroperitoneum. In C. M. Rumack & D. Levine (Eds.), Diagnostic ultrasound (5th ed., pp. 432-469). Elsevier.
- Daye, D., & Walker, T. (2018). Complications of endovascular aneurysm repair of the thoracic and abdominal aorta: evaluation and management. Cardiovascular Diagnosis and Therapy, 8(S1), S138-S156. doi: 10.21037/cdt.2017.09.17 Faiza, Z., & Sharman, T. (2023). Thoracic aorta aneurysm. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK554567/
- Gao, J., Cao, H., Hu, G., Wu, Y., Xu, Y., Cui, H., Lu, H. S., & Zheng, L. (2023). The mechanism and therapy of aortic aneurysms. Signal Transduction and Targeted Therapy, 8 (55), 1-20. https://doi.org/10.1038/s41392-023-01325-7
- Golledge, J. (2019). Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nature Reviews Cardiology, 16, 225-245. https://doi.org/10.1038/ s41569-018-0114-9
- Joviliano, E. E., Ribeiro, M. S., & Tenorio, E. J. R. (2017). MicroRNAs and current concepts on the pathogenesis of abdominal aortic aneurysm. Brazilian Journal of Cardiovascular Surgery, 32(3), 215-224. https://doi.org/10.21470/1678-9741-2016-0050
- Kassem, T. W. (2017). Follow up CT angiography post EVAR: Endoleaks detection, classification and management planning. The Egyptian Journal of Radiology and Nuclear Medicine, 48(3), 621-626. doi: 10.1016/j.ejrnm.2017.03.025
- Pandey, N., & Litt, H. (2015). Surveillance Imaging Following Endovascular Aneurysm Repair. Seminars In Interventional Radiology, 32(3), 239-248. doi: 10.1055/s-0035-1556878
- Robinson, D., Mees, B., Verhagen, H., Chuen, J. (2013). Aortic aneurysms screening, surveillance and referral. Australian Family Physician, 42(6). https://www.racgp.org.au/afp/2013/june/aortic-aneurysms Shaw, P. M., Loree, J., & Gibbons, R. C. (2023). Abdominal aortic aneurysm. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK470237/
- Theivendran, M. & Chuen, J. (2018). Updates on AAA screening and surveillance. Australian Journal of General Practice, 47(5). doi: 0.31128/AFP-07-17-4278 Wise, E. S., Hocking, K. M., & Brophy, C. M. (2016). Prediction of in-hospital mortality after ruptured abdominal aortic aneurysm repair using an artificial neural network. Journal of Vascular Surgery, 62(1), 8-15. doi: 10.1016/j.jvs.2015.02.038
