24th August 2024, A/Prof Chee L Khoo

Last fortnight we looked at the effects of hypertriglyceridaemia (HTG) on atherosclerosis. We explored the source and metabolism of triglycerides (TG). We saw how important lipoprotein lipase was in regulating plasma TG. Triglyceride levels are closely related to plasma triglycerides rich lipoprotein (TRL) and TRL remnants. All three components penetrate through the endothelium into the subendothelial space where atherosclerotic inflammation occur. We also looked at the numerous secondary causes of HTG. In this issue, we examine the options for the treatment of HTG.
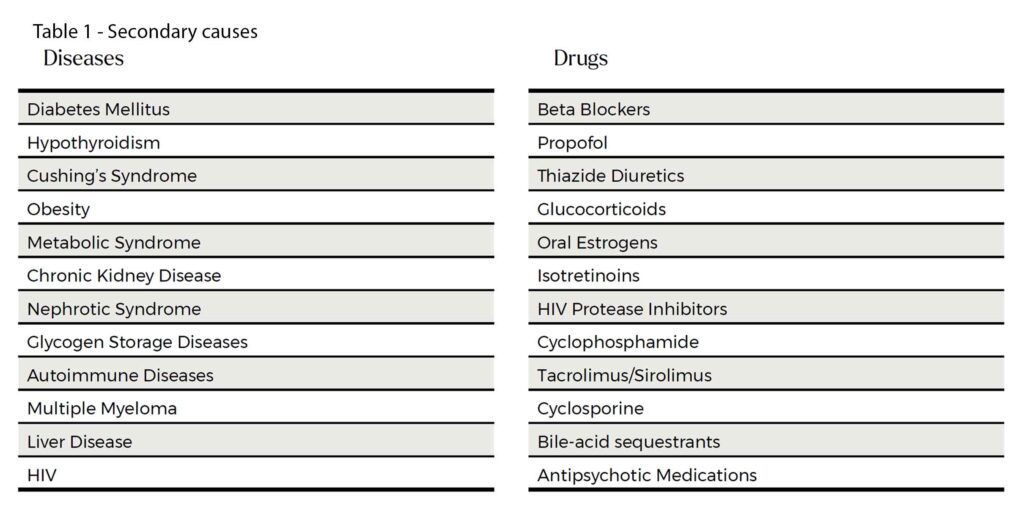
It is worth relooking at the long list of secondary causes. See Table 1. The list looks long but in practice, the usual suspects are obesity, high carbohydrate diet, suboptimal glycaemic control, excess alcohol intake and hypothyroidism. The common culprit drugs are corticosteroids, psychotropics, isotretinoins and some commonly used chemotherapy drugs (tacrolimus, cyclophosphamide and cyclosporine).
Primary causes of HTG are mainly polygenic and typically, the triglyceride levels are extreme (>5-10 mmol/L). These patients not only have high risks of pancreatitis but have high cardiovascular risks involving multiple vascular beds. They warrant treatment of the TG to prevent pancreatitis, referral to the local genetic clinic, a review of all vascular beds and aggressive management of the cardiovascular risks.
Treatment of hypertriglycerides
Lifestyle Measures
Many of the secondary causes are lifestyle related. All patients with HTG should adopt healthy lifestyles which include weight loss, calorie restriction, alcohol intake reduction and regular exercise. A 5-10% weight reduction can lead to an ~ 20% decrease in TG levels. To achieve that we will need calorie restriction. Dietary fatty acid is a significant source of TRL. Restriction of saturated fats, refined sugars, and simple carbohydrates are essential. High protein diets including lean meats, poultry, and plant-based proteins are preferred to red meat (1). Aerobic and endurance exercise can decrease triglyceride levels by as much as 30% (1,2). Exercise increases fatty acid use, reduces post prandial triglyceride concentrations, and reduces fat stores (1,3-5).
Statins
Although we use statins primarily to reduce cholesterol (especially LDL-C), statins reduce triglycerides levels by 10-30%. The reduction is greater in patients with higher the baseline TG. Unfortunately, reducing triglycerides in isolation have not been consistently associated with reduction in CV events. Reducing triglycerides in patients whose triglycerides are >1.7 mmol/L despite statins have been shown to reduce CV risks. In practice, if you have a patient with high triglycerides, you may want to ask whether this patient should be on statin,
Fibrates
Fibrates work by increasing the activity of peroxisome proliferator-activated receptor-α(PPAR α). Fibrates include fenofibrate, gemfibrozil, bezafibrate, ciprofibrate, and pemafibrate. Bezafibrate, ciprofibrate, and pemafibrate are not available in Australia. Gemfibrozil has too many side effects. Patients with severe HTG (>5.6 mmol/L) are at risk of acute pancreatitis and if lifestyle measures have been attended to, fibrate therapy is indicated to reduce the risk of pancreatitis. The aim of fibrate therapy is to reduce acute pancreatitis and this is in patients with TG > 5.6 mmol/L. In patients with mild to moderate HTG (<5.6 mol/L), fibrate therapy is not indicated. What about CV risk reduction in these patients? Unfortunately, studies have not shown reduction in CV mortality with fenofibrate.
In the Helsinki Heart Study (HHS) and the Veterans Affairs High-Density Lipoprotein Intervention Trial (VA-HIT), patients not on statins showed improvements in composite CV outcomes with fibrates (34% in HHS and 22% in VA-HIT but in the era of statin use, the results cannot be extended to the patients we see in our practice (8-10).
In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, patients were on statins and were randomised to fenofibrate or placebo (11). Despite a larger reduction in TG in the fenofibrate group, there were no difference in CV outcomes. There was a suggestion in a sub-analysis that there may be CV benefit in a subgroup of patients with both elevated TG and low HDL-C. The Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) trial explored that idea (12). Nope. Despite triglyceride improvement, pemafibrate did not reduce the primary CV outcome.
This is not to be mistaken for microvascular benefits (retinopathy) in patients with diabetes. Both ACCORD and FIELD yielded signals for microvascular benefit.
Niacin
Niacin can reduce TG levels by 20-50% but once again, a number of trials have failed to demonstrate CV benefits (HPS2-THRIVE, AIM-HIGH) (13,14). Further, niacin has too many nasty side effects like worsening hyperglycaemia, flushing, and hepatotoxicity.
Omega-3 fatty acids
There has been many clinical trials studying combination of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) as well as clinical trials using purified EPA. Irrespective of the doses used, O3FA are very effective in reducing TG. But does it help reduce CV outcomes?
Most of the clinical trials used combination formulations of EPA and DHA:
- Outcome Reduction with an Initial Glargine Intervention (ORIGIN) (13)
- A Study of Cardiovascular Events in Diabetes (ASCEND) (14)
- Vitamin D and Omega-3 Trial (VITAL) (15), OMega-3 fatty acids in Elderly patients with Myocardial Infarction (OMEMI) (16) and
- Long-Term Outcomes Study to Assess Statin Residual Risk with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia (STRENGTH) (17)
Most, but not all, of the subjects in these trials are on statins. They all reduce triglyceride levels but none of the above trials demonstrated any CV benefits.
Pure EPA Omega-3 Fatty Acids
Not all Omega-3 fatty acids are the same. The action of EPA and DHA on lipid metabolism and the pro-inflammatory milieu are not the same. Perhaps, EPA only trial might provide CV benefits.
In the Japan EPA Lipid Intervention Study (JELIS) 18,645 Japanese patients with total cholesterol ≥6.5 mmol/L who were randomised to 1.8 mg/day EPA and statin compared with statin only (18). After a mean follow-up of 4.6 years, the EPA group had significantly lower rates of the composite CV endpoint of major adverse cardiovascular events (MACE) (2.8% vs 3.5%, P=0.01). The EPA formulation used in the trial was Epadel which contain highly purified (>98%) fish-derived ethyl EPA.
In the Combination Therapy of Eicosapentaenoic Acid and Pitavastatin for Coronary Plaque Regression Evaluated by Integrated Backscatter Intravascular Ultrasonography (CHERRY) and the Effect of Vascepa on Improving Coronary Atherosclerosis in People With High Triglycerides Taking Statin Therapy (EVAPORATE) trials, icosapent ethyl which is a pure EPA preparation Plus statin were shown to reduce coronary plaque (demonstrated on intravascular US or CT coronary angiography) (19-20). Similarly, in the Randomized trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy – Statin and EPA (RESPECT-EPA), icosapent ethyl showed a trend towards improvement in the composite outcome of CV death, MI, stroke, unstable angina, and revascularization (9.1% vs 10.6%, P=0.055) (21).
The strongest evidence of the effect of pure EPA on CV events is from the REDUCE-IT trial. 8,179 patients from 11 different countries with high CV risk on statin therapy who had triglyceride levels of 1.5-5.6 mmol/L and LDL-C of 1.1-2.6 mmol/L were randomised to 4g/day of iscosapent ethyl or mineral oil placebo (22). After 4.9 years, there was a 25% reduction in the primary composite outcome of CV death, nonfatal MI, nonfatal stroke, coronary revascularization, or unstable angina. There were higher rates of atrial fibrillation or atrial flutter hospitalisations (3.1% vs 2.1%, P=0.004) and a trend towards higher rates of serious bleeding events (2.7% vs 2.1%, P=0.06) in the icosapent ethyl compared with placebo group.
The future in hypertriglyceridaemia management
Because TG levels are largely influenced by the action of Lipoprotein Lipase (LPL), a number of agents have been tested in clinical trials. LPL causes lipolysis of TRL and angiopoietin-like protein 3 (ANGPTL3), angiopoietin-like protein 4 (ANGPTL4) and apolipoprotein C3 (apoCIII) all inhibit LPL and leads to elevation of TG. Novel agents in development target these inhibitors.
Olezarsen is an antisense oligonucleotide that inhibits apoCIII messenger RNA (mRNA). In patients with familial chylomicronemia syndrome, olezarsen showed significant reduction in triglyceride levels and in frequency of acute pancreatitis. Plozasiran is a small interfering RNA (siRNA) which has demonstrated efficacy in reducing TG in patients with severe HTG and mixed hyperlipidaemia.
Evinacumab, zodasiran, and solbinsiran all inhibit the ANGPTL3 and have been demonstrated to reduce TG in patients with severe HTG. From their names, you can guess that evinacumab is a monoclonal antibody while the other two are siRNAs.
The novel agents have demonstrated efficacy in reducing TG levels which is most useful in those patients with severe HTG. This can lead to reduction in episodes of acute pancreatitis. Whether these agents can reduce CV events in patients with hypertriglyceridemia is yet to be established.
In summary, patients with severe hypertriglyceridaemia (>5.6mmol/L) are at risk of pancreatitis and currently treatment consist of lifestyle measures and fenofibrate. In these patients and in patients with lesser degrees of HTG, fenofibrate has not been shown to reduce cardiovascular risks. Marine sourced omega-3 fatty acids can either be combination EPA and DHA or pure EPA. Most commercial, over the counter fish oil capsules have combination EPA/DHA omega3 fish oils. Combination omega-3 fish oil therapy have been shown to effectively reduce triglyceride levels but have not led to reduction in cardiovascular events. Only trials using purified EPA (icosapent ethyl) have demonstrated CV reduction. Patients in these trials are also on statins. The exact mechanisms for the benefit is yet to be elucidated but it is likely to involve more than TG lowering alone.
Thus, treatment of patients with high triglycerides involved lifestyle measures, aggressive treatment of the associated CV risks (with maximal statins +/- ezetimibe +/- PCSK9 therapy) and then to treat any residual hypertriglyceridaemia. It’s not treatment of the triglycerides per se but treatment of the CV risks that shows benefits. Icosapent ethyl (Vazkepa®) has been TGA approved since November 2022. By the time, this article goes to air, Vazkepa is likely to be available in Australia.
References:
- Virani SS, Morris PB, Agarwala A, Ballantyne CM, et al. 2021 ACC Expert Consensus Decision Pathway on the Management of ASCVD Risk Reduction in Patients With Persistent Hypertriglyceridemia: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;78:960–993.
- Martin WH. Effects of acute and chronic exercise on fat metabolism. Exerc Sport Sci Rev. 1996;24:203–231.
- Koutsari C, Karpe F, Humphreys SM, Frayn KN, Hardman AE. Exercise prevents the accumulation of triglyceride-rich lipoproteins and their remnants seen when changing to a high-carbohydrate diet. Arterioscler Thromb Vasc Biol. 2001;21:1520–1525.
- Byrne A, Makadia S, Sutherland A, Miller M. Optimizing Non-Pharmacologic Management of Hypertriglyceridemia. Arch Med Res. 2017;48:483–487.
- Kushner PA, Cobble ME. Hypertriglyceridemia: the importance of identifying patients at risk. Postgrad Med. 2016;128:848–858.
- Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245.
- Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418.
- Bloomfield Rubins H, Davenport J, Babikian V, VA-HIT Study Group. Reduction in stroke with gemfibrozil in men with coronary heart disease and low HDL cholesterol: The Veterans Affairs HDL Intervention Trial (VA-HIT). Circulation. 2001;103:2828–2833.
- ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574.
- Das Pradhan A, Glynn RJ, Fruchart J-C, MacFadyen JGet al, PROMINENT Investigators. Triglyceride Lowering with Pemafibrate to Reduce Cardiovascular Risk. N Engl J Med. 2022;387:1923–1934.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–1291.
- AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267.
- ORIGIN Trial Investigators, Bosch J, Gerstein HC, Dagenais GR, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–318.
- ASCEND Study Collaborative Group, Bowman L, Mafham M, Wallendszus K, et al. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N Engl J Med. 2018;379:1540–1550.
- Manson JE, Bassuk SS, Buring JE, VITAL Research Group. Principal results of the VITamin D and OmegA-3 TriaL (VITAL) and updated meta-analyses of relevant vitamin D trials. J Steroid Biochem Mol Biol. 2020;198:105522.
- Kalstad AA, Myhre PL, Laake K, et al. OMEMI Investigators. Effects of n-3 Fatty Acid Supplements in Elderly Patients After Myocardial Infarction: A Randomized, Controlled Trial. Circulation. 2021;143:528–539.
- Nicholls SJ, Lincoff AM, Garcia M, ET AL. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA. 2020;324:2268–2280.
- Yokoyama M, Origasa H, Matsuzaki M, et al. Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet Lond Engl. 2007;369:1090–1098.
- Watanabe T, Ando K, Daidoji H, ET AL, CHERRY study investigators. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol. 2017;70:537–544.
- Budoff MJ, Bhatt DL, Kinninger A, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. 2020;41:3925–3932.
- Miyauchi K, Iwata H, Nishizaki Y, et al. RESPECT-EPA Investigators. Randomized Trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy-Statin and Eicosapentaenoic Acid (RESPECT-EPA). Circulation. 2024;
- Bhatt DL, Steg PG, Miller M, et al. REDUCE-IT Investigators. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019;380:11–22.
