13th October 2024, A/Prof Chee L Khoo
We dissected the latest consensus statement from GESA in the last issue of GPVoice when we looked at metabolic dysfunction related fatty liver disease (MAFLD). We highlighted the risk groups that are very likely to have MAFLD. Essentially, these are patients with metabolic syndrome (type 2 diabetes, obesity, hypertriglyceridaemia and hypertension). We looked at the recommendations of using ultrasonography as the first line investigation to diagnose MAFLD and not to rely on abnormal liver function tests (LFTs) to rule in or rule out MAFLD. We described how to use FIB-4 score to decide whether the patient need further investigations and referral or can be managed in primary care. As promised, we will look at how to safely monitor these patients in primary care for progression of fibrosis and development of hepatocellular carcinoma (HCC).
Metabolic dysfunction is not the only cause of MAFLD. Other causes should be considered when the diagnosis of MAFLD is made. Of course, there can be more than one cause. See Box 1. The more causes there are, the higher the risk of developing liver and non-liver complications.

An elevated serum ALT level of >40 U/L for men and >35 U/L for women110 should trigger assessment of risk factors for chronic hepatitis B and C infection.
What are monitoring?
Patients with MAFLD are not only at higher risk of developing liver complications (cirrhosis and HCC) but they are also at higher risk of cardiovascular disease and chronic kidney disease and likely to have obstructive sleep apnoea (OSA). As good primary care physicians, we should conduct a thorough cardiovascular (CV) risk assessment, assess renal function and been suspicious of OSA and treat them aggressively.
Fortunately, the progression of liver fibrosis in patients with MAFLD is relatively slow. In a meta-analysis of 54 observational studies and clinical trials involving 26,738 people, the average time to progress one fibrosis stage in those with no or minimal fibrosis (F0–1) is 10 years (1). It is likely that we will seeing these patients quite frequently and regularly in our practice. However, 6%–15% of people with F0–1 fibrosis progressing to advanced fibrosis or cirrhosis (F3–4) within 5 years (1).
Serial FIB-4s
We already know how easy it is to use FIB-4 to “quantify” the severity of liver fibrosis and to determine who needs further investigations and referral. Well, we can as easily use serial FIB-4 scores to monitor progression. Small increment in the score is not significant but an increase in FIB-4 score from low-risk to above indeterminate– or high-risk thresholds over a 3-year period is associated with an increased risk of incident cirrhosis, HCC and liver related death (2,3).
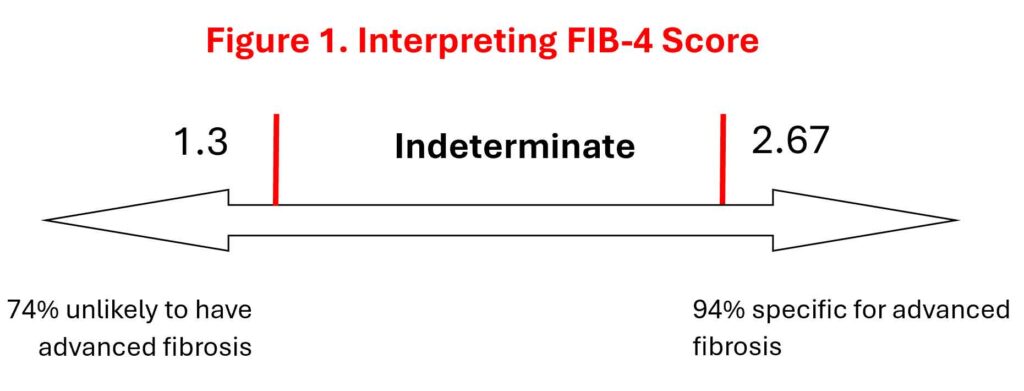
The incidence of cirrhosis or HCC in patients with a persistently low FIB-4 score was 0.4/1000 person years but for those transitioning from low to indeterminate scores the incidence increased to 1.3/1000 person-years. For those transitioning from low to high scores the incidence ballooned up to 8.6/1000 person-years (2).
Liver function tests (LFTs) and HbA1c
A 10-point increase in AST (but not ALT) is associated with a 30% increased risk of fibrosis progression. Not unexpectedly, patients with T2D are more likely to have fibrosis progression compared with those without (4). A 1% increase in HbA1c is associated with 15% higher odds of an increase in fibrosis stage (5,6).
Older patients
The prevalence of MAFLD reduces with age and for those over 70-75 years old who has MAFLD but without advanced fibrosis, the mortality rate is not increased. However, in those with MAFLD and advanced fibrosis, it’s a different story. These patients continue to have higher mortality and higher risks of incident HCC and liver related complications. They need to monitored like the other younger patients.
Recommendations
For people who are 75 years or older and have MAFLD, routine monitoring for fibrosis progression should be performed on a case-by-case basis, depending on their coexisting conditions and life expectancy.
What about HCC?
70-80% of HCC develop in patients with MAFLD with cirrhosis (F4). Looking from the other angle, in a meta-analysis of 18 observational studies involving 470,404 patients, the annual rate of HCC development in patients with cirrhotic MAFLD was > 3.5% (7). Thus, identifying patients who has cirrhotic MAFLD is paramount and these patients need surveillance for HCC development. Nothing is never in medicine. The annual risk of HCC development in non-cirrhotic HCC is <0.05% and HCC surveillance in this group impractical (7,8).
HCC surveillance is performed with liver-directed ultrasound, with or without measurement of serum alpha-fetoprotein (AFP) levels, every 6 months and should be coordinated in conjunction with a specialist with expertise in liver disease. Remember, patients with advanced fibrosis should be referred to a specialist with expertise in liver disease. Small liver nodules (<10mm) need more frequent (3 monthly) ultrasound to monitor for growth.
Of course, the reason and benefit of HCC surveillance is that the early detection of small HCCs that are more amenable to curative therapies. However, surveillance needs to be seen from the view of future mortality risk. HCC surveillance is recommended for people with Child–Pugh A or B cirrhosis, those with Child–Pugh C cirrhosis who are potential liver transplantation candidates and those without life-limiting comorbidities and reasonable functional status (9).
In summary, there are a lot more MAFLD in primary care than you think. As mentioned, there is a huge under diagnosis. Many of these patients may already have significant liver fibrosis and warrant referral to specialist services. MAFLD without significant can be easily and safely monitored and managed in primary care but the trick is to find those who need to be referred. Up to 30% of people with MAFLD assessed in primary care have a FIB-4 score greater than 1.3 and require further assessment, whereas the prevalence of advanced fibrosis in general practice is only 5%–10%.
References:
- Le P, Payne JY, Zhang L, et al. Disease state transition probabilities across the spectrum of NAFLD: a systematic review and meta-analysis of paired biopsy or imaging studies. Clin Gastroenterol Hepatol 2023; 21: 1154-1168.
- Cholankeril G, Kramer JR, Chu J, et al. Longitudinal changes in fibrosis markers are associated with risk of cirrhosis and hepatocellular carcinoma in non-alcoholic fatty liver disease. J Hepatol 2023; 78: 493-500.
- Hagstrom H, Talback M, Andreasson A, et al. Repeated FIB-4 measurements can help identify individuals at risk of severe liver disease. J Hepatol 2020; 73: 1023-1029.
- Huang DQ, Wilson LA, Behling C, et al. Fibrosis progression rate in biopsy-proven nonalcoholic fatty liver disease among people with diabetes versus people without diabetes: a multicenter study. Gastroenterology 2023; 165: 463-472.e5.
- Kleiner DE, Brunt EM, Wilson LA, et al. Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open 2019; 2: e1912565.
- Alexopoulos AS, Crowley MJ, Wang Y, et al. Glycemic control predicts severity of hepatocyte ballooning and hepatic fibrosis in nonalcoholic fatty liver disease. Hepatology 2021; 74: 1220-1233.
- Orci LA, Sanduzzi-Zamparelli M, Caballol B, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin Gastroenterol Hepatol 2022; 20:283-292.e10.
- Singal AG, El-Serag HB. Rational HCC screening approaches for patients with NAFLD. J Hepatol 2022; 76: 195-201.
- Taddei TH, Jou JH. When to stop screening—liver cancer. Am J Gastroenterol 2023; 118: 427-428.
- https://www.gesa.org.au/public/13/files/Education%20%26%20Resources/Clinical%20Practice%20Resources/MAFLD/MAFLD%20consensus%20statement%202024.pdf
