18th November 2024, A/Prof Chee L Khoo
Amongst the many causes of heart failure with preserved ejection fraction (HFpEF) is obesity. This is not surprising because obesity is now considered an inflammatory chronic disease. Increase adiposity leads to increased released of pro-inflammatory cytokines which damages myocardial muscles. It would then follow that reducing that fat mass would reduce the pro-inflammatory environment and result in lower incidence of HFpEF as well as reducing complications of HF. We have already seen the positive results from the STEP-HFpEF trial that weekly semaglutide 2.4mg reduce the risk of cardiovascular death and worsening heart-failure events. Now is tirzepatide’s turn to report on HFpEF.
Tirzepatide is a dual GIP/GLP1 agonist in one molecule. GIP has the advantage of specifically increasing blood flow to the adipocytes, improving adipocyte insulin sensitivity and improving lipid metabolism. This, theoretically, means that tirzepatide should lead to better improvement in the inflammatory environment and thence, better effect on HFpEF than just losing weight.
The SUMMIT trial is a double-blind, randomised, placebo-controlled trial looking to examine effect of tirzepatide on worsening heart failure events, health status, and functional capacity in patients with HFpEF. The results were published this week in the New England Journal of Medicine (1).
Between April 20, 2021, and June 30, 2023, a total of 1494 patients were screened, and 731 patients were randomly assigned to receive tirzepatide (364 patients) or placebo (367 patients) at 129 centres in nine countries. Subjects had to be >40 years old, have NYHA Class II to IV symptomatic heart failure, left ventricular EF of ≥ 50% and BMI ≥ 30.
For the diagnosis of HFpEF, they need to have at least one of the following:
- Elevated NT-proBNP level (defined as >200 pg per ml in patients with sinus rhythm or >600 pg per ml in patients with atrial fibrillation),
- Left atrial enlargement (assessed on two-dimensional echocardiography), or
- Elevated filling pressures at rest or during exercise (assessed by invasive or noninvasive measurements).
Patients were also required to have had heart-failure decompensation within 12 months before baseline or to have an eGFR < 70 ml/min/1.73 m2 at baseline.
To assess physical function, patients had a 6-minute walk distance of between 100 and 425 m and a Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CSS) of 80 or lower (scores range from 0 to 100, with higher scores indicating better quality of life).
Patients in the intervention arm started with 2.5mg weekly tirzepatide which was titrated up monthly till they reached 15mg weekly by 20 weeks and this was continued to the end of the trial. Patients were evaluated every 1-6 months and the 6-minute walk distance, KCCQ-CSS, and high-sensitivity C-reactive protein (CRP) level were assessed at baseline and at 24 and 52 weeks.
The primary composite end point was death from cardiovascular causes or a worsening heart-failure event over a median duration of 104 weeks. Worsening heart-failure event was defined as exacerbated symptoms of heart failure resulting in hospitalisation, intravenous therapy in an urgent care setting, or intensification of oral diuretic therapy.
The results
The primary outcome (death from cardiovascular causes or a worsening heart-failure event) occurred in 9.9% of the tirzepatide group vs 15.3% of the placebo group (a hazard ratio of 0.62, p=0.026). If you remove CV deaths from the outcomes, then the hazard ratio for a worsening heart-failure event was 0.54 (95% CI, 0.34 to 0.85) and for a worsening heart-failure event resulting in hospitalisation was 0.44 (95% CI, 0.22 to 0.87. If we remove intensification of oral diuretic from the worsening heart failure event, the hazard ratio was 0.57.
Overall, there were 15 cardiovascular deaths but 11 of them were not preceded by worsening of heart failure. In other words, it is unlikely that HF killed them. 2 of the deaths occurred in patients who stopped their tirzepatide for more than 15 months. Death from any cause occurred in 19 patients in tirzepatide group and in 15 patients in the placebo group (hazard ratio, 1.25; 95% CI, 0.63 to 2.45).
Functional improvement
At 52 weeks, the mean increase in the KCCQCSS was 19.5 points in the tirzepatide group and 12.7 points in the placebo group (between-group median difference, 6.9).
Secondary endpoints
Not surprisingly, patients on tirzepatide lost -13.9% body weight vs -2.2% in the placebo group. The mean increase in the 6-minute walk distance was 26.0 m in the tirzepatide group and 10.1 m in the placebo group. The mean reduction in HS-CRP was 38.8% in the tirzepatide group and -5.9% in the placebo group.
There were no differences in the number of serious adverse events between the groups.
What does the study results mean?
This was a heart failure trial with very obese subjects and the improvement in worsening heart failure was massive. On worsening of heart failure alone, there was between 38-56% improvement in tirzepatide. It took them 3 months to get to the maximal dose of 15mg of tirzepatide. If we look at the graph, the benefit appeared very shortly after at about 6 months.
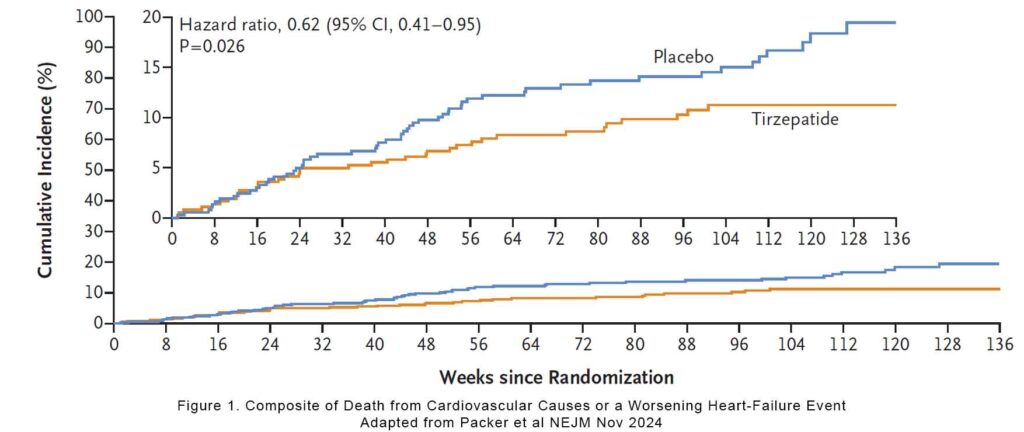
Unlike the STEP-HFpEF trial, not all the patients in this trial needed to have an elevated NT-proBNP (2). It illustrates that many of our patients with HFpEF may not have elevated NT-proBNP. They may have other features of structural abnormalities and symptoms consistent with heart failure. Remember, NT-proBNP is very useful in ruling heart failure (<125) but borderline NT-proBNP may still indicate heart failure. The median NT-proBNP in this study was 200 pg/ml.
The use of BMI > 30 as an inclusion criteria may exclude many patients who have high visceral fat but have BMI < 30. A waist-hip ratio may be a more reliable indicator of visceral fat.
In summary, not unexpected, weekly tirzepatide 15mg led to significant weight loss in obese patients with HFpEF. The incidence of worsening of heart failure was significantly reduced (38-56%) in these patients. Incretin therapy (GLP1-RA+/-GIP agonist) will have to be incorporated into guidelines in the treatment of HFpEF soon.
References:
- Milton Packer, Michael R. Zile, Christopher M. Kramer, et al. Tirzepatide for Heart Failure with Preserved Ejection Fraction and Obesity. NEJM Nov 16, 2024
- Kosiborod MN, Abildstrøm SZ, Borlaug BA, et L. STEP-HFpEF Trial Committees and Investigators. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N Engl J Med. 2023 Sep 21;389(12):1069-1084.
