29th October 2018, Dr Chee L Khoo
Incretins are hormones released when we eat. They augment the release of insulin from pancreatic beta cells. They not only lead to reduction in blood glucose levels via the secretion of insulin, they also inhibit glucagon release thereby stemming further hyperglycaemia, slow the absorption of nutrients by slowing gastric emptying and increase satiety. The two main incretin hormones are glucagon like peptide -1 (GLP1) and gastric inhibitory peptides (GIP). There are now 7 GLP approved for use in the management of hyperglycaemia in type 2 diabetes (T2D) but what about the GIP? Why haven’t we heard about them? Why don’t we have GIP analogues? Well, we do. Read on.
They both work on the beta cell to release insulin in response to hyperglycaemia. They work by binding to specific G protein-coupled receptor (GPCR) on the beta cell surface which activates cAMP leading to insulin release. The GLP1 specific GPCR are present in islet cells (beta, delta and possibly alpha cells), CNS, GI Tract, kidneys, lungs and heart. The GIP specific GPCR, on the other hand are found primarily in islet cells, CNS and adipose tissues.
Why should there be two incretins? Interestingly, in mice experiments, if you delete the GLP1 receptors, GIP secretion is augmented. Similarly, if we delete the GIP receptors, GLP1 secretion is augmented. Interestingly, healthy human subjects, Michael Nauck et al (JCEM 1993) showed that the combination of both GIP and GLP1 together stimulates beta cell secretion more than either incretins alone. In other words, two incretins are better than one. However, other studies suggest that GIP actually don’t stimulate beta cell that well and it also stimulate alpha cell glucagon secretion.
So, what happens when you combine both a GLP1 agonist with a GIP agonist in pharmacological doses? LY3298176 is a novel dual GLP1 and GIP receptor agonist. It activates both GLP1 and GIP receptors in islet cells. In healthy human subjects, LY3298176 caused weight loss and improved glucose tolerance. In subjects with T2D, LY3298176 reduced fasting glucose, glucose excursions and body weight with increasing doses.
LY3298176 is 39 amino acid peptide based on the native GIP peptide 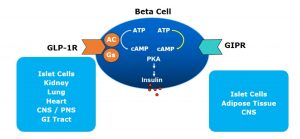
modified to bind to both GLP1 and GIP receptors. It is more potent than native GIP but less potent than native GLP1. It has a half-life of 5 days allowing for a weekly dosing.
In a 26 week randomised, placebo- and Active Comparator-controlled trial, LY3298176 in increasing doses were compared with dulaglutide and placebo. The primary objective was the dose response of LY3298176 relative to placebo at 26 weeks. Secondary objectives were the effect on body weight, percentage of patients achieving HbA1c under 7.0% and percentage of patients achieving >5% or 10% weight loss at 26 weeks.
HbA1c efficacy
At 26 weeks, dulaglutide reduced HbA1c by 1.1%. The lowest dose of LY3298176 (1mg) reduced HbA1c by 0.7% while the highest dose (15mg) reduced HbA1c by a whopping 2.4%. 51.9% of patients on dulaglutide achieved HbA1c <7.0% while 10mg of LY3298176 got 90% of patients under HbA1c <7.0%.
Weight loss
At 26 weeks, patients on dulaglutide lost on average 2.7 kg. Patients on the highest dose lost a whopping 11.3 kg. 22.2% of patients on dulaglutide achieved >5% weight loss while 70% of patients on 10mg LY3298176 achieved >5% weight loss. The proportion who lost >10% weight loss were 9.3% for dulaglutide and 37.7% for LY3298176.

Adverse events
Incidence of nausea, vomiting and diarrhoea increased with increasing doses of LY3298176.
The results of the clinical trial was first presented in Berlin at the EASD 2018 meeting. LY3298176 is looking like a very promising treatment option for patients with T2D. It’s coming real soon.
