13th November 2018, Dr Chee L Khoo
It’s finally here. The Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE–TIMI 58) trial finally released their results this week at the American Heart Association (AHA) Scientific Sessions 2018. The results were most impressive but this was not just another CV outcome trial of another SGLT2 inhibitors. The results have major implications in the way we treat patients with type 2 diabetes (T2D) in general practice.
DECLARE-TIMI 58 was a large (17,160 patients) trial of dapagliflozin in patients with T2D and either established ASCVD or multiple risk factors for ASCVD. 17,160 patients with T2D were evaluated. 10,186 of those patients did not have established CV disease which is an important point because the study replicates what we see in general practice. Patients were followed up for 4.2 years, much longer than the other SGLT2 trials (EMPA-REG and CANVAS).
The primary outcome was a composite of major adverse cardiovascular events (MACE), defined as cardiovascular death, myocardial infarction, or ischemic stroke and a composite of cardiovascular death or hospitalisation for heart failure. Secondary outcomes were a renal composite of ≥40% decrease in estimated glomerular filtration rate (eGFR), new end-stage renal disease, or death from renal or cardiovascular causes and death from any cause.
What did they find?
Remember, this is a trial of patients with T2D and a mixed of cardiovascular risks – some with established cardiac disease (40%) and some just had risk factors (60%). After 4.2 years, if we looked at the composite of major adverese cardiovascular events dapagliflozin was non-inferior to placebo.
After the groups were separated into patients with and those without established cardiovascular disease, MACE were non-significantly reduced with dapagliflozin in those with established disease, but there was no effect in those without established CVD.
But if we looked into just cardiovascular death and hospitalisation for health failure, there was a 17% reduction with dapagliflozin. Most of the benefit came from reduction in heart failure (27% reduction). Interestingly, most patients did not have a history of heart failure at baseline. There were no difference in cardiovascular deaths between the groups.
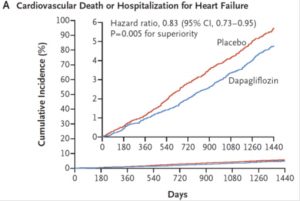
Adapted from NEJM Nov 2018
There was a 23% reduction in renal composite outcome in the dapagliflozin compared with placebo. Death from any cause did not differ between groups.
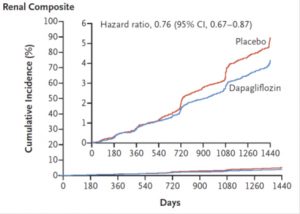
Adapted from NEJM Nov 2018
Adverse events
In the EMPA-REG study, empagliflozin was associated with remarkable reduction of cardiovascular morbidity and mortality and all-cause death. But there was a slight increase in the incidence of stroke although the numbers did not reached statistical significance. SGLT2 inhibitors are known to be associated with increased in haemocrit and it is thought that this increase in haematocrit may be a possible cause in the increase in stroke incidence. In CANVAS, canagliflozin was also associated with a two-fold increased risk for lower limb amputations.
In this study, dapagliflozin was not associated with a higher risk of stroke, amputations, or fractures. Dapagliflozin group had more genital infections and diabetes ketoacidosis but less bladder cancer compared with placebo.
Implications of DECLARE-TIMI 58
DECLARE-TIMI 58 adds to previous SGLT2 inhibitor studies which demonstrated beneficial effects on MACE in patients with established heart disease. In addition, DECLARE-TIMI 58 have demonstrated that even in patients without establish heart disease but with multiple cardiovascular risk, dapagliflozin can reduce hospitalisation for heart failure.
Dapagliflozin also reduce renal events. There was a consistent pattern of lower rates of progression of renal disease was seen among patients with and those without established atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease at baseline.
The subjects in this study was more representative of the general practice patients than the subjects in EMPA-REG, CANVAS and LEADER studies which only looked at patients with established heart disease.
Access the abstract here.
Reference
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117-28.
Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study). Circulation 2018;137:323-334.
S.D. Wiviott, I. Raz, M.P. Bonaca,et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med Nov 18, 2018
