27th January 2019, Dr Chee L Khoo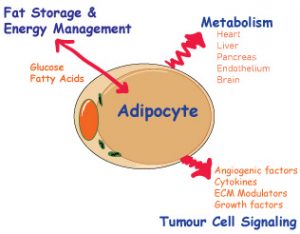
Energy consumption, regulation and storage vital for the survival of the organism. We have specialised cells that manages the above but often, the cells do a bit of each depending on the energy needs of the organism at the time. When you think about it, the most effective cell to do all of the above is actually the adipocyte. Over the last couple of decades, the role of the adipocyte is revealed to be more than just storing and releasing energy.
There is ongoing debate about the definition of “healthy adipose tissue’” or whether there is such a thing as healthy obese. One of the most intriguing questions about the adipocyte has to be why some adipocytes remain “healthy” while others become “unhealthy”. The health of the adipocyte is definitely not related to the actual fat mass. So, when does adipose tissues become “unhealthy”?
The classical role of the adipocyte is the cell that soaks up fatty acids and glucose after meals and hydrolyse and releases triacylglycerols in the fasting state. The adipocyte is now considered to be an endocrine organ releasing peptides, metabolites and signalling lipids, all of which is now understood to be important in inter-organ communication.
So, what happens to the adipose tissues in obesity? When the body’s fat mass increases, the number of fat cells increases. The existing fat cells also increase in size. So, you have more fatter fat cells. Given that adipose tissue can grow vastly in size, adapting to the increase demand for nutrients can be challenging.
Like any organ in the body, the critical limiting factor when adipose tissue expand is blood supply. Adipose tissue requires proper delivery of essential nutrients and oxygen; therefore, optimal vascular function is critical. Increasing vascular density stimulates adipose tissue to undergo healthy expansion. The increased vascularisation also serves as an important source for mesenchymal stem cells, which are recruited to adipose tissue to become pre-adipocytes. These pre-adipocytes are subsequently activated and embark on a full adipogenic program.
Inadequate vascularisation, on the other hand, leads to hypoxia. When adipocytes are under distress (hypoxia), inflammatory cytokines are released. Hypoxia induced factors ultimately lead to fibrosis in the adipose tissues. These inflammatory factors also influence the nature of the immune response resulting in a pro-inflammatory state. These immune changes not only exist in the adipose tissues but effect system wide changes. Other organs including kidneys, heart, brain and joint tissues are affected by the pro-inflammatory state.
The hallmarks of healthy adipose tissue are as follows:
- High degree of vascular density within the fat depot
- Minimal hypoxia and fibrosis; and
- Low level of macrophage infiltration into adipose tissue and a relatively low level of inflammation.
In addition to hypoxia induced factors, an increasing number of adipokines have been identified and studied – adipsin, adiponectin, leptin, angiogenic growth factors, extra-cellular matrix modulators. Adiponectin and leptin are the most widely studied. Extensive cross talk between different cells and organs have been identified. The still somewhat mysterious connection between increased BMI and cancer incidence has only partially been unravelled, particularly for a subset of cancers, which include endometrial cancers, post-menopausal breast cancers, pancreatic cancers and colon cancers.
It is traditionally thought that adipocyte can either 1) increase in size 2) decrease in size or 3) die (apoptosis). There is now ample evidence that the adipocyte can undergo de-differentiation by reverting back to a pre-adipocyte, a fibroblast or myofibroblast. For example, during late pregnancy and lactation, adipocytes undergo full de-differentiation (to transform into lactating cells) only to differentiate back to adipocytes upon cessation of lactation.
The conventional notions regarding fat distribution still hold true. A fat distribution that leans towards a more central visceral location (i.e. the ‘apple’ shape), is associated with a less favourable metabolic phenotype. A distribution where the subcutaneous fat depot is preferentially expanded, particularly in the lower extremities (i.e. the ‘pear’ shape), is associated with a more favourable metabolic phenotype.
Adipocytes also come in different colours – white, beige or brown. Compared with the white adipocytes, brown adipocytes contain multiple small drops of lipids and more mitochondria. They contain more capillaries and supply the tissue with nutrients. Brown adipocytes are abundant in newborn (for thermogenesis) but its prevalence decreases with age. The white adipocytes in the subcutaneous fat can recruit beige adipocytes (which metabolically more active cells) in response to cold and beta adrenergic receptor agonist stimulation.
Given the central role the adipocyte plays in systemic metabolic homeostasis, as well as Inter-organ signalling including tumour cell interactions, adipocyte is potential frug target. Watch this space.
Reference:
Scherer, P.E. Diabetologia (2019) 62: 223. https://doi.org/10.1007/s00125-018-4777-x
