26th July 2019, Dr Chee L Khoo
All of us remember the association of QT prolongation with the dramatic Torsale de Pointes (TdP) and sudden cardiac death (SCD) from medical school. Fortunately, both are relatively rare in general practice and we really don’t need to know much about QT prolongation in general practice as it belongs to the hospital people and the cardiologists, right? Unfortunately, there are increasing number of common drugs that we use in general practice including SSRIs, antibiotics and beta-blockers and increasing number of medical conditions that we encounter in general practice that are associated with prolongation of the QT interval and we do need to know about this QT beast.
To understand the patho-physiology of long QT syndrome (LQTS), we need to revise the physiology of myocyte depolarisation:
At rest (polarised)
There is a resting action potential across the membrane of cardiac muscles. There are lots of sodium (Na+) outside the cells and lots of potassium (K+) inside the cells. There are relatively more positive charged ions outside the cells. The cell is said to be polarised. The Na+/K+-ATPase pump is closed.
Depolarisation
The Na+/K+ pump opens allowing a rush of Na+ in and K+ out of the cells. For every 2 Na+ ions that goes in there are 3 K+ ions that goes out. This leads to a change in the charge inside the cell from negative (-ve) to positive (+ve). The cell is now depolarised. The Na+/K+ATPase pump closes. The change in polarity then allows calcium (Ca2+) to rush in through the voltage–gated calcium channels (VGCCs). The cardiac muscle contracts.
Repolarisation
Calcium channels closes. Na+ is pumped out of the cell in exchange for K+ pumping into the cells. The resting action poten
tial across the membrane (-ve inside and +ve outside) is restored. The cell is now repolarised.
Prolonged repolarisation
QT prolongation is the result from prolongation of repolarisation. Usually, when a new action potential is generated (following repolarisation), the myocytes are in a refractory state. The prolongation of repolarisation may result in subsequent activation of an inward depolarisation current, known as an early after-depolarisation, which may promote triggered activity. Re-entry, due to a dispersion of refractory periods, is also possible. As soon as the mid myocardial layer is no longer in a refractory period, excitation from nearby tissue will cause a retrograde current and a re-entry circuit that will result in a positive chronotropic cycle, leading to tachycardia.
LQTS can either be caused from genetic abnormalities or secondary to electrolyte disturbances, structurally cardiac abnormalities or drug interactions.
Inherited causes
The most common genetic abnormality is the Romano-Ward Syndrome affecting between 1:2000 to 1:7000 people worldwide. Other less common inherited long QT syndromes are Jervell and Lange-Nielsen syndrome, Andersen-Tawil syndrome and Timothy syndrome.
Non-inherited causes
The common causes that we might encounter in general practice are:
- Electrolyte disturbances – Hypokalaemia, Hypomagnesaemia, Hypocalcaemia
- Hypoglycaemia
- Heart failure
- Bradycardia
- Hypothyroidism
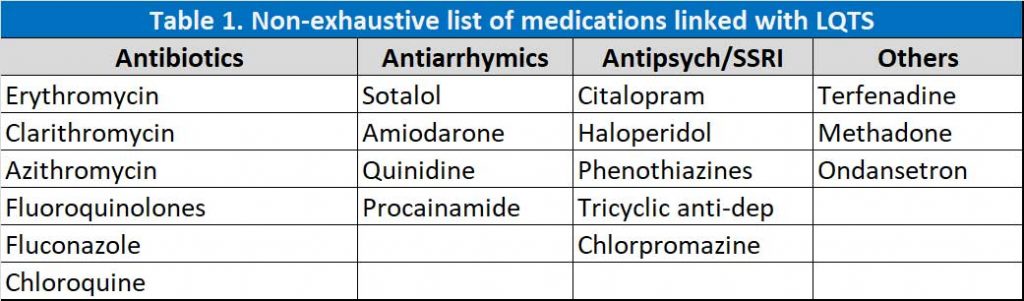
- Drug interactions (see Table 1)
We still have a few problems which we need to overcome – how to measure the QT interval and determining who is at risk of arrhythmia associated with LQTS.
Measuring QT interval 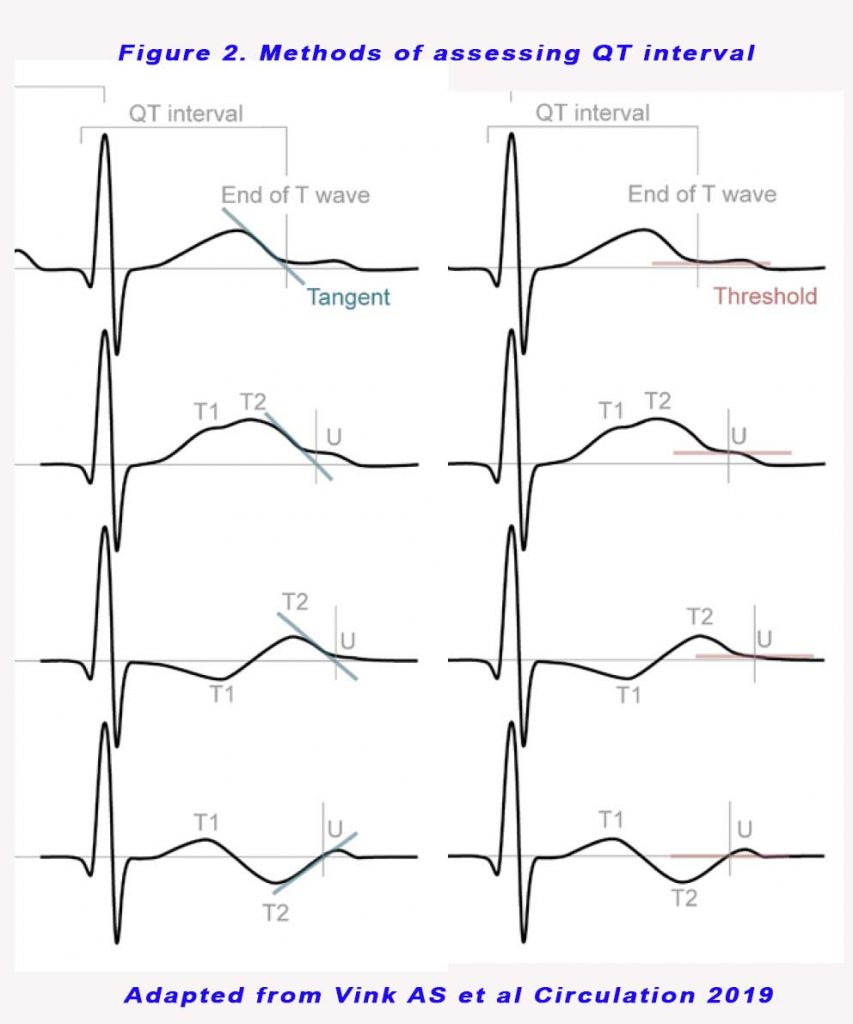
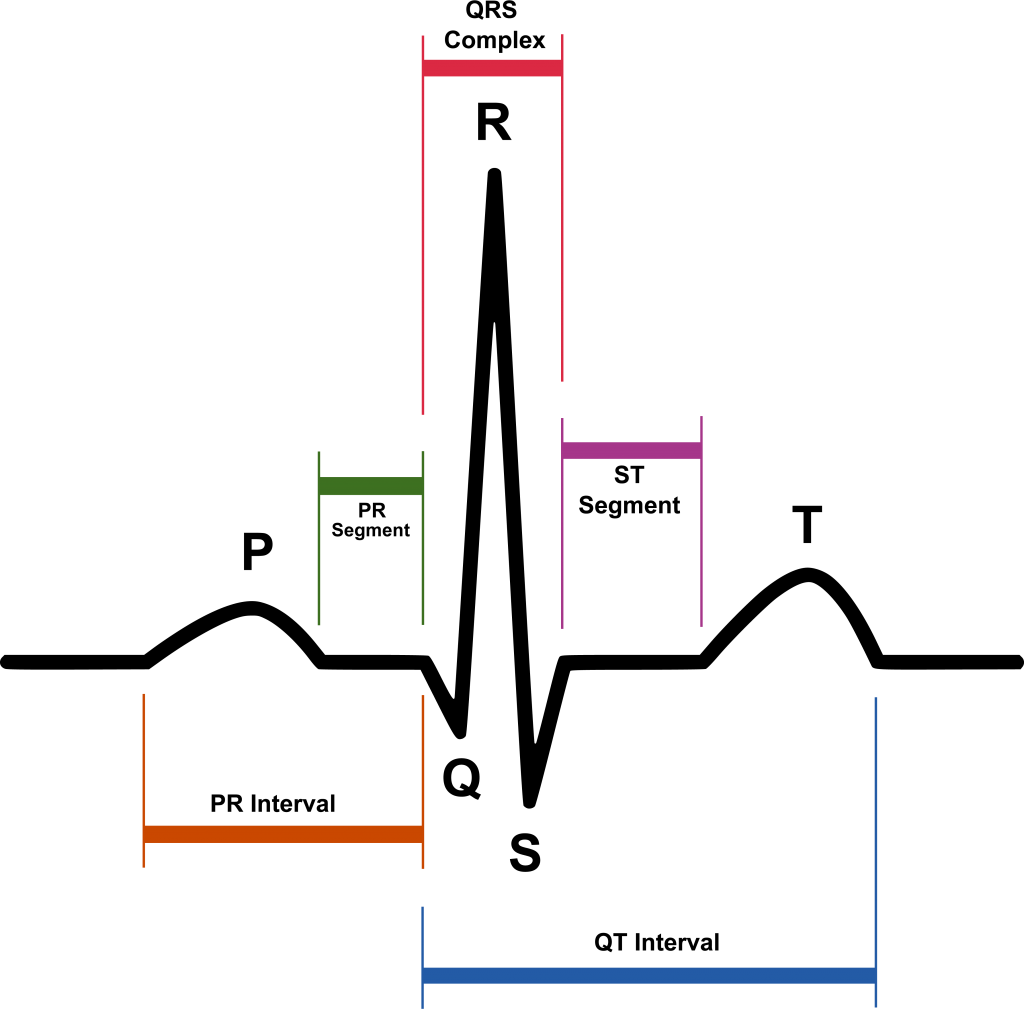
The QT interval is calculated as the time from the start of the Q wave to the end of the T wave, and approximates to the time taken from when the cardiac ventricles start to contract to when they finish relaxing (see Figure 1). The QT interval needs to be corrected for heart rate. There are 2 methods for manual QT interval assessments – the tangential and the threshold method (see Figure 2). The second component of the T wave (T2) was always included in the QT interval, and a U wave was always excluded from QT interval analyses. For the threshold method, when a U wave interrupted the T wave before it returned to baseline, the end of the QT interval was defined as the nadir between the T and U wave.
The cut off values for both methods of QT measurements are obviously different. This has large implications on the diagnosis and management.
A heart rate corrected QT interval, QTc is defined as a QTc value >450 ms in males and >460 ms in females measured preferably in lead II or V5 on a standard 12-lead ECG, predisposes to functional reentry, torsades de pointes, and sudden cardiac death and represents a widely accepted, easily measured, and quantifiable risk factor for adverse outcomes (1,2) An otherwise unexplained baseline QTc >500 ms should prompt clinical suspicion for the possible presence of a potentially lethal, highly treatable condition known as congenital long-QT syndrome (2,3). Despite the importance of the degree of QT interval prolongation, no standard method for its measurement and correction for heart rate has been unequivocally established.
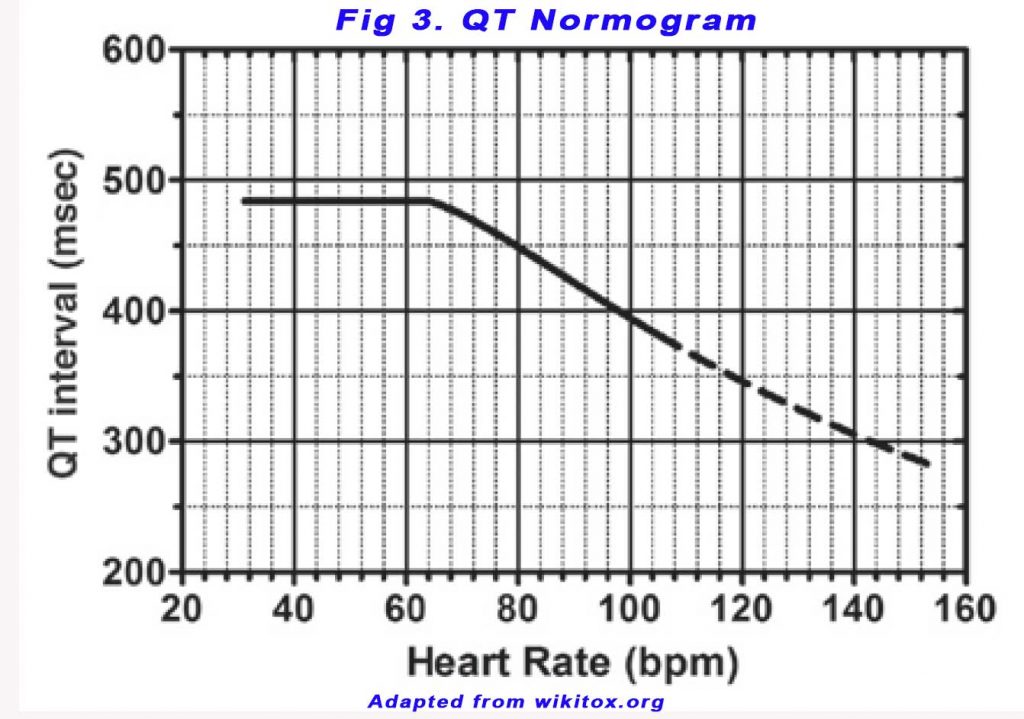
Readouts from automated ECGs are unreliable and QT intervals should be measured manually. The QT nomogram, a plot of QT interval versus heart rate, can be used as a risk assessment tool to detect an abnormal QT interval (see Figure 3). A freely accessible online probability-calculator for LQTS (www.QTcalculator.org) has been made available to provide further guidance in appropriate QT interval assessment for users worldwide (4).
Arrhythmia suppression involves the use of medications or surgical procedures that attack the underlying cause of the arrhythmias associated with LQTS. Since the cause of arrhythmias in LQTS is early after depolarisations, and they are increased in states of adrenergic stimulation, steps can be taken to blunt adrenergic stimulation in these individuals. These include administration of beta receptor blocking agents, which decreases the risk of stress-induced arrhythmias.
Relevance to general practice
It is now thought that a prolonged QTc value, whether secondary to genetics, drugs, electrolyte perturbations, or systemic disease, represents a critical barometer of the heart’s electric system capable of identifying potentially at-risk individuals. Evidence suggest that even modest QTc prolongation in middle-aged and older adults may serve as an early marker for serious cardiovascular events and death (1).
When prescribing drugs that prolong the QT interval, the balance of benefit versus harm should always be considered. Before prescribing a drug that causes QT prolongation and torsades de pointes, it is essential to undertake a baseline assessment. A reasonable minimum would be a single baseline ECG. This initial assessment establishes if the patient has an abnormal QT interval ‘off’ the drug which would contraindicate the use of a QT-prolonging drug. If the patient is commenced on the drug, they require serial ECGs during treatment to check for QT prolongation. It is important to avoid other causes of QT prolongation such as electrolyte abnormalities and hypoglycaemia during at illness.
SCD accounts for 230,000 to 350,000 deaths per year in the United States. While many who suffer SCD possess underlying structural heart disease, inherited arrhythmia syndromes are also important contributors to SCD. In patients without structural heart disease, inherited arrhythmia syndromes are identified in >50% of the remaining patients.
In general practice, this is the golden rule:
*Don’t allow 2 causes of prolonged QT in the one patient.*
1) If a patient has congenital prolonged QT (or less commonly prolonged QT due to electrolyte disturbance, cardiac ischaemia or failure), then don’t prescribe any drugs which further prolong the QT interval, OR
2) If the patient is on 1 drug which prolongs the QT, then don’t co-prescribe a 2nd drug which prolongs the QT. Prolonged QT is associated with ventricular arrhythmias which can cause sudden cardiac death.
LQTS is largely asymptomatic. Should we be assessing the QTc interval in patients about to embark on high level sports?
References:
- Beinart R, Zhang Y, Lima JA, Bluemke DA, Soliman EZ, Heckbert SR, Post WS, Guallar E, Nazarian S. The QT interval is associated with incident cardiovascular events: the MESA study. J Am Coll Cardiol. 2014;64:2111–2119. doi: 10.1016/j.jacc.2014.08.039
- Vink AS, Neumann B, Lieve KVV, Sinner MF, Hofman N, El Kadi S, Schoenmaker MHA, Slaghekke HMJ, de Jong JSSG, Clur SB, Blom NA, Kääb S, Wilde AAM, Postema PG. determination and interpretation of the QT interval. Circulation. 2018;138:2345–2358. doi: 10.1161/CIRCULATIONAHA. 118.033943
- Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, Philippides GJ, Roden DM, Zareba W; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology, the Council on Cardiovascular Nursing, and the American College of Cardiology Foundation. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121:1047–1060. doi: 10.1161/CIRCULATIONAHA.109.192704
- Arja Suzanne Vink, Benjamin Neumann, Krystien V.V. Lieve, et al. Determination and Interpretation of the QT Interval Comprehensive Analysis of a Large Cohort of Long QT Syndrome Patients and Controls. Circulation. 2018;138:2345–2358. DOI: 10.1161/CIRCULATIONAHA.118.033943
- By Created by Agateller (Anthony Atkielski), converted to svg by atom. – SinusRhythmLabels.png, Public Domain, https://commons.wikimedia.org/w/index.php?curid=1560893
- By PeaBrainC – Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=74185800
