14th December 2020, Dr Chee L Khoo
We explored the Covid-19 vaccine candidates in the last issue. You would have read that the Pfizer vaccine has been approved and has been rolled out in UK and US. I have yet to come across the full disclosure of the trial anywhere. The Oxford/AstraZeneca trial has been published in full and it is worthwhile dissecting the study and ponder the implications as other vaccines are being rolled out internationally and will hit Australian shores in early 2021. What impact will the vaccine have when we get them? Who will NOT be putting their hands up for the vaccine? What is the marketing plan if we were to attempt to vaccinate “everyone”?
The Oxford/AstraZeneca covid-19 vaccine is a replication-deficient chimpanzee adenoviral vector
ChAdOx1, containing the SARS-CoV-2 structural surface glycoprotein antigen (so-called spike protein) gene. It’s big mouthful and it’s easy to cut and paste the whole phrase but what does that even mean? Adenovirus is a common cold virus. It is commonly used to carry a payload of genetic material to human cells. The problem is that because human adenovirus infection is pretty common, most people will have immunity against human adenovirus. Using human adenovirus as a vector risk the vaccine being targeted by our immune response before the payload is delivered.
ChAdOx1 is a chimpanzee adenovirus that has been genetically modified so that it is impossible to grow in human cells. Chimpanzee adenoviral vectors are a very well-studied vaccine type, having been used safely in thousands of subjects. It has been shown to generate strong immune response after vaccination. By the way, the Russian Sputnik V vaccine uses a human adenovirus as the vector.
The Oxford COVID-19 vaccine team is led by Prof Sarah Gilbert, Prof Andrew Pollard, Prof Teresa Lambe, Dr Sandy Douglas, Prof Catherine Green and Prof Adrian Hill. They had already used ChAdOx1 vaccine technology to produce candidate vaccines against flu, Zika and Middle East Respiratory Syndrome (MERS). Work had already begun when the pandemic emerged from China. As soon as the genetic sequence was available, they began work on a trial. The work was not new nor rushed.
The ChAdOx1 vaccine contains the genetic sequence of this surface spike protein. When the vaccine enters cells inside the body, it uses this genetic code to produce the surface spike protein of the coronavirus. This induces an immune response, priming the immune system to attack the coronavirus if it later infects the body.
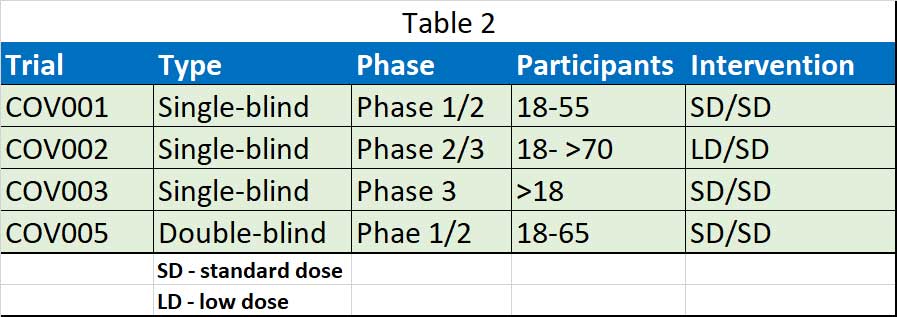
The Oxford vaccine team released their first clinical efficacy results of ChAdOx1 nCoV-19 in a pooled analysis of phase 2/3 trials in the UK and Brazil, and safety data from more than 20 000 participants enrolled across four clinical trials in the UK, Brazil, and South Africa. They include data from four ongoing blinded, randomised, controlled trials done across three countries: COV001 (phase 1/2; UK), COV002 (phase 2/3; UK), COV003 (phase 3; Brazil), and COV005 (phase 1/2; South Africa) (see Table 1). All four studies included participants who received two doses, with a booster dose incorporated into the three trials that were initially designed to assess a single-dose of ChAdOx1 nCoV-19 compared with control after review of the antibody response data.
Participants across all four trials were asked to contact the study site if they experienced specific symptoms associated with COVID-19. Those who met symptomatic criteria had a clinical assessment, a swab taken for a nucleic acid amplification test (NAAT), and blood samples taken for safety and immunogenicity.
Participants were excluded from the primary efficacy analysis if they were seropositive at baseline or had no baseline result. Other exclusions included those with NAAT-positive swabs within 14 days after the second vaccination, or those who discontinued from the study before having met the primary efficacy endpoint with a follow-up time of less than 15 days after the second vaccination.
For symptomatic participants in COV002 in the UK, weekly swabbing continued both before and after participants reported symptoms to the study site. To test for asymptomatic infections, participants in COV002 in the UK were asked to provide a weekly self-administered nose and throat swab for NAAT testing from 1 week after first vaccination. In Brazil, there was no testing plan for asymptomatic infections. In South Africa, asymptomatic infections were detected from swabs obtained at study visits attended.
Outcomes
Pimary outcome was virologically confirmed, symptomatic COVID-19, defined as a NAAT-positive swab combined with at least one qualifying symptom (fever =37·8°C, cough, shortness of breath, or anosmia or ageusia). Between April 23 and Nov 4, 2020, 23 848 participants were recruited and vaccinated across the four studies. Those aged >56 years were recruited later and contributed 12·2% of the total cohort in the current analysis.
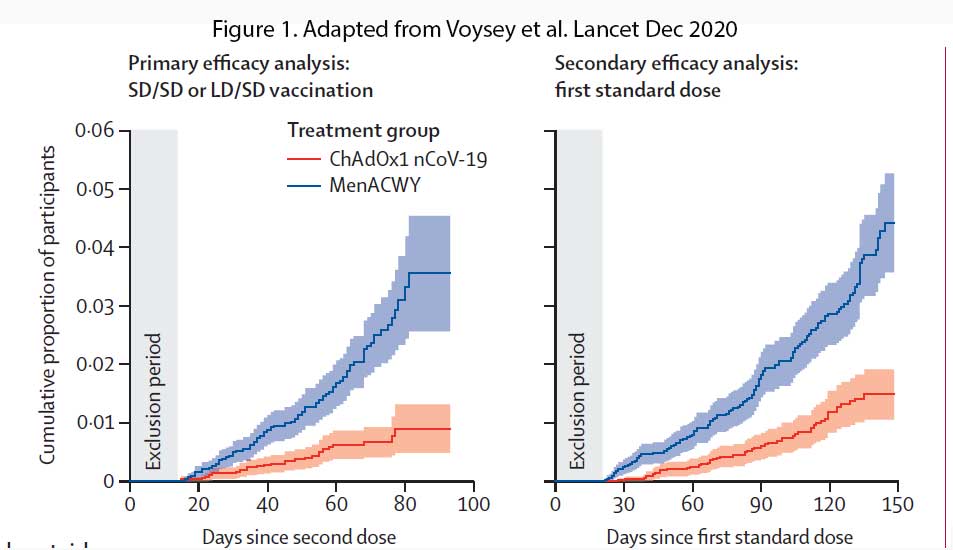
In participants who received two standard-dose vaccines, vaccine efficacy was 62.1% (95% CI 41.0–75.7), whereas in those who received a low dose as their first dose of vaccine, efficacy was higher at 90.0% (see Figure 1). Overall vaccine efficacy across both groups was 70.4% (95・8% CI 54.8–80.6; 30 [0・5%] of 5807 vs 101 [1.7%] of 5829). From 21 days after the first dose, there were ten cases hospitalised for COVID-19, all in the control arm; two were classified as severe COVID-19, including one death. There were 74 341 person-months of safety follow-up.
Adverse events
Serious adverse events occurred in 168 participants, 79 of whom received ChAdOx1 nCoV-19 and 89 of whom received MenACWY or saline control. A case of haemolytic anaemia in the control group in the UK phase 1/2 study occurring 10 days after MenACWY vaccine was considered possibly related to the intervention and has been previously described. 14 days after ChAdOx1 nCoV-19 booster vaccination as being possibly related to vaccination, with the independent neurological committee considering the most likely diagnosis to be of an idiopathic
A case of transverse myelitis was reported 14 days after ChAdOx1 nCoV-19 booster vaccination as being possibly related to vaccination, with the independent neurological committee considering the most likely diagnosis to be of an idiopathic. A potentially vaccine-related serious adverse event was reported 2 days after vaccination in South Africa in an individual who recorded fever higher than 40°C, but who recovered rapidly without an alternative diagnosis and was not admitted to hospital.
ChAdOx1 nCoV-19 has an acceptable safety profile and is efficacious against symptomatic COVID-19, with no hospital admissions or severe cases reported in the ChAdOx1 nCoV-19 arm. The vaccine can be stored and distributed at 2–8°C, making it particularly suitable for global distribution.
Does the vaccine change anything in Australia?
The US and Europe have failed to control the pandemic in their countries – the virus have overwhelmed the hospital capacity to cope, they have no effective tracing working, by and large they are under resourced in their testing capacity and their lock down attempts are either half hearted or not effective. They have lost control and have no other cards left. The vaccine is the only step left to slow down the pandemic and save lives. If replicated across the population and if the take up rate is >60-70%, they might be able to succeed in achieving those bold aims.
But wait. The ChAdOx1 nCoV-19 vaccine is only claiming efficacy against symptomatic Covid-19 infections. Which means people who has the vaccine will still get Covid-19 but will either be asymptomatic or have mild symptoms and less likely to be hospitalised and less likely to die. They can still pass on the virus to someone else who is not immune or vaccinated.
The situation in Australia (and New Zealand) is very different. We have control of the pandemic. We have minimal daily new cases. We have small number of deaths. Our lock down measures has been successful. We have an amazing tracing capacity. We still have ample hospital capacity should there be an upswing in cases in the near future. Not sure what a successful vaccination campaign will achieve when the vaccine arrives in March 2021, assuming the take up rate is >70% is achieved.
We have yet to see the full results of the other two candidates – the Moderna and the Pfizer vaccines but preliminary indications are that they are claiming the same – efficacy against symptomatic infections.
References:
Voysey M, Clemens SAC, Madhi SA, et al: Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2020 Dec 8:S0140-6736(20)32661-1. doi: 10.1016/S0140-6736(20)32661-1. Epub ahead of print. PMID: 33306989; PMCID: PMC7723445.
