26th February 2021, Dr Chee L Khoo
Back in medical school, we were taught that we have B-cells and T-cells. B-cells are responsible for humoral immunity and T-cells are responsible for cell-mediated immunity. T-cells could be killer cells or helper cells although we also have regulatory T-cells which tells the naïve T-cells how to behave. Of course, over the last 2-3 decades, we know more about what makes the regulatory T-cells decide what to tell these naïve cells. All this new knowledge make the treatment of auto-immune diseases very targeted and very specific. One of the commonly used disease-modifying agents in autoimmune arthropathies are the JAK inhibitors. I am not sure about you but I have no idea what these increasingly and commonly used agents are.
Back to basics
The T helper cells (Th cells), also known as CD4+ cells, as their name suggests, “help” the activity of other immune cells by releasing cytokines that alter the behaviour of target cells that express receptors for those cytokines. These cells help to determine the appropriate immune response depending on the nature of the immunological insult (virus vs. extracellular bacterium vs. intracellular bacterium vs. helminth vs. fungus vs. protist). They are generally considered important in modifying B cell behaviour and in the activation and growth of cytotoxic T cells. They also modulate the bactericidal activity of macrophages and neutrophils. Essentially, Th cells are pro-inflammatory cells.
Activation and suppression of Th cells
Antigen-presenting cells (APCs) are primarily dendritic cells, macrophages and B cells. During an immune response, APCs endocytose antigens (typically bacteria or viruses), undergo processing, then travel from the infection site to the lymph nodes. The antigen on the APC is presented to the Th cells, the Th cells are activated and they differentiate into effector T-cells. Which effector T-cells they differentiate into is determined by the antigen presented – Th 1 helper cells lead to an increased cell-mediated response, typically against intracellular bacteria and protozoa while Th 2 helper cells lead to a humoral immune response, typically against extracellular parasites including helminths.
As you have already guessed, if there is an activation system of Th cells, there must be an opposing suppression system of Th cells. The regulatory T cells (Treg cells), formerly known as suppressor T cells, are a subpopulation of T cells that modulate the immune system, maintain tolerance to self-antigens, and prevent autoimmune disease. Tregs are immunosuppressive and suppress or downregulate induction and proliferation of effector T cells.
The Interleukin-23/Interleukin-17 pathway
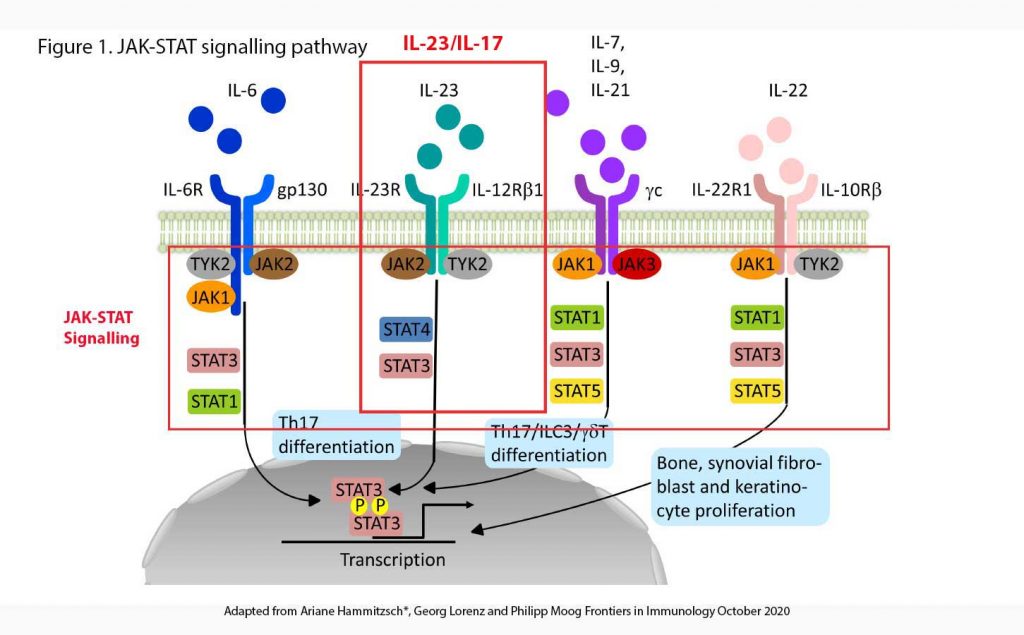
Many cytokines (amongst them interleukins) have been identified over the last few decades which further our understanding of the immune response to antigens, internal or external (see Figure 1). In the Interleukin-23 (IL-23) pathway, after APCs are activated by antigens, IL-23 is released. IL-23 binds on to IL-23 receptors (IL-23R) on the surface of effector T-cells (including γδ T-cells, innate lymphoid cells, natural killer cells, macrophages). This, in turn trigger a series of intracellular events involving JAK, TYK and STAT (see below) and ultimately, result in the release of a number of cytokines including interleukin-17 (IL-17), interleukin-22 (IL-22), tumour necrotic factor-α (TNF-α) and TNF-γ.
IL-17 trigger the conversion of naïve Th cells into pathogenic Th17 cells. Th17 cells have been shown to be necessary for maintenance of mucosal immunity. The dysregulation of Th17 cells has been associated with autoimmune disorders and inflammation. In the case of autoimmune disorders, Th17 cell over activation can cause an inappropriate amount of inflammation, like in the case of auto-immune arthropathy.
Despite the protective role played by the IL-23/IL-17 axis against bacterial and fungal infections, its dysregulation contributes to chronic inflammation and the development of several autoimmune diseases like psoriasis, psoriatic arthritis, ankylosing spondylitis (AS), inflammatory bowel disease, rheumatoid arthritis (RA), Sjogren syndrome and multiple sclerosis.
The JAK-STAT signalling pathway
JAK and STAT are central intracellular signal transducers for a great number of pro-inflammatory (e.g. IL-2, IL-7, IL-12, and IL-23) and anti-inflammatory cytokines (e.g. IL-10) influencing the immune responses thought to be essential for the induction of AS. The intracellular tyrosine kinase family consists of JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2) and is coupled to STAT molecules (STAT1, STAT2, STAT3, STAT4, STAT5a and b, and STAT6) (7).
Therapeutics
Advances in understanding the pathogenesis of AS (and other arthropathies) have prompted the development of biologic drugs designed to inhibit the IL-23/IL-17 axis. For more than 15 years, TNF-a inhibitors (e.g. etanercept (Enbrel), adalimumab (Humira)) were the only biologic treatment available and, despite an initial great success in AS management, almost 40% of patients failed to reach a significant response.
The new therapeutic agents interfering with the IL-23/IL-17 axis can be divided into monoclonal antibodies directly targeting IL-17, IL-23 or their receptors and JAK inhibitors (or so-called JAKinibs) inhibiting the intracellular pathways triggered by these cytokines. Some of the -mabs which target IL-17 include Secukinumab (Cosentyx), Ixekizumab (Taltz), Netakimab and Bimekizumab (). IL-23 targeting drugs have been less successful in demonstrating efficacy against autoimmune arthorpathies. See Figure 2.
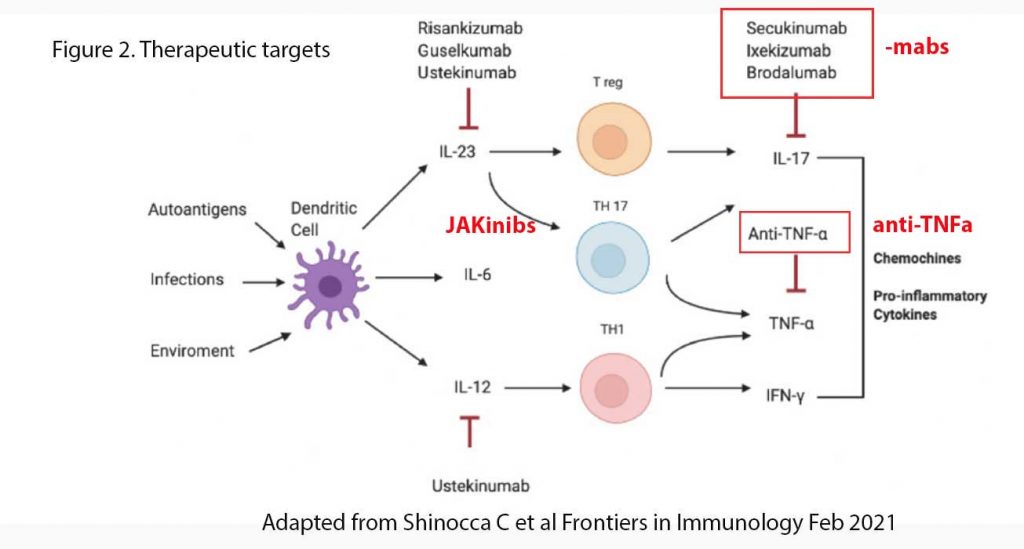
Some of the more commonly used JAKinibs are Tofacitinib (Xeljanz®), a pan-JAK inhibitor, Filgotinib (Jyseleca®) and Upadacitinib (Rinvoq®), both JAK1 inhibitors, were shown to be superior to placebo in various auto-immune arthropathies including AS and RA.
Future perspectives to implement the therapeutic options for AS patients include broader anti-inflammatory approaches with multi-cytokine blockade to overcome the inhibition of a single pathway, for example targeting simultaneously TNF-a and IL-17, as in an ongoing study in psoriatic arthropathy.
References:
- Hammitzsch A, Lorenz G, Moog P. Impact of Janus Kinase Inhibition on the Treatment of Axial Spondyloarthropathies. Front Immunol. 2020 Oct 21;11:591176. doi: 10.3389/fimmu.2020.591176. PMID: 33193430; PMCID: PMC7609840.
- Schinocca C, Rizzo C, Fasano S, Grasso G, La Barbera L, Ciccia F and Guggino G (2021). Role of the IL-23/IL-17 Pathway in Rheumatic Diseases: An Overview. Front. Immunol. 12:637829. doi: 10.3389/fimmu.2021.637829
- https:/ /en.wikipedia.org/wiki/Interleukin_23 Accessed 24th February 2021
- https:/ /en.wikipedia.org/wiki/T_helper_cell Accessed 24th February 2021
- https://en.wikipedia.org/wiki/T_helper_17_cell Accessed 24th February 2021
