12th March 2021, Dr Chee L Khoo

I don’t about you but I seem to be bombarded by emails championing the wonderful things that cannabis oil can do. I even had a pharmaceutical rep swinging by to ask why I wasn’t prescribing cannabis oil for my patients. I even have a specialist (of sorts) that recommended that my patient come and discuss cannabis oil for his condition with me. Over the past 5 years, public interest in the potential health benefits of cannabis oil has increased exponentially, and a wide range of over-the-counter preparations of cannabis oil are now available. A substantial proportion of the population appears to have used these products, yet the extent to which they are effective or safe is unclear. How should we respond when patients coming knocking asking for advice whether they should try these products?
Let’s clear up some terminology. Cannabis sativa is one of the world’s most versatile crops. Cannabis can be separated into two distinct categories, specifically ‘hemp’ and ‘marijuana’. The difference between hemp and marijuana is the amount of tetrahydrocannabinol (THC) present in the plant. To be considered hemp, cannabis must possess a low percentage of THC relative to the total dry weight of flowers but the THC percentage vary from country to country. In order to be legally cultivated as hemp, the cultivated plants must possess less than 0.3% THC (w/w) in Canada and China, whereas since 2001, the European Union determined that the THC content (w/w) of hemp must be below 0.2%.
There is at least >400 known compounds in the cannabis plant including THC and at least >100 other cannabinoids. Apart from THC, cannabidiol (CBD) is one of the most studied cannabinoids. CBD oil or medicinal marijuana or cannabis oil is concentrated CBD as the active ingredient but without the THC. CBD can be taken in multiple ways, including by inhalation of cannabis smoke or vapor, as an aerosol spray into the cheek and by mouth.
Mechanisms of action
Two cannabinoid receptors, CB1 and CB2, were identified in early 1990s. Interestingly, the identification of these two CB receptors subsequently led to the discovery of two endogenous receptor ligand – arachidonyl-ethanolamide (anandamide ligand) and 2-arachidonoyl-glycerol (2-AG) which binds to the CB receptors. CB receptors are primarily localised to areas responsible for:
(1) higher cognitive function
(2) movement
(3) control of sensory and motor functions of the autonomic nervous system [52].
The action of CBD is complex and include:
- Agonism of transient receptor potential (TRP) channels
- Partial agonism of dopamine D2 receptors and serotonin 5-HT1A receptors
- Positive allosteric modulation of μ and δ-opioid receptors
- Inhibition of adenosine reuptake
- Competitive antagonism of G protein-coupled receptor 55 (GPR55)
- Inhibition of inflammatory cytokines via peroxisome proliferator-activated receptor-gamma (PPARγ) receptors
- Negative allosteric modulator at CB1 and CB2 receptors, reducing their response to agonists such as Δ9-THC, anandamide and 2-arachidonoylglycerol.
See Table 1.
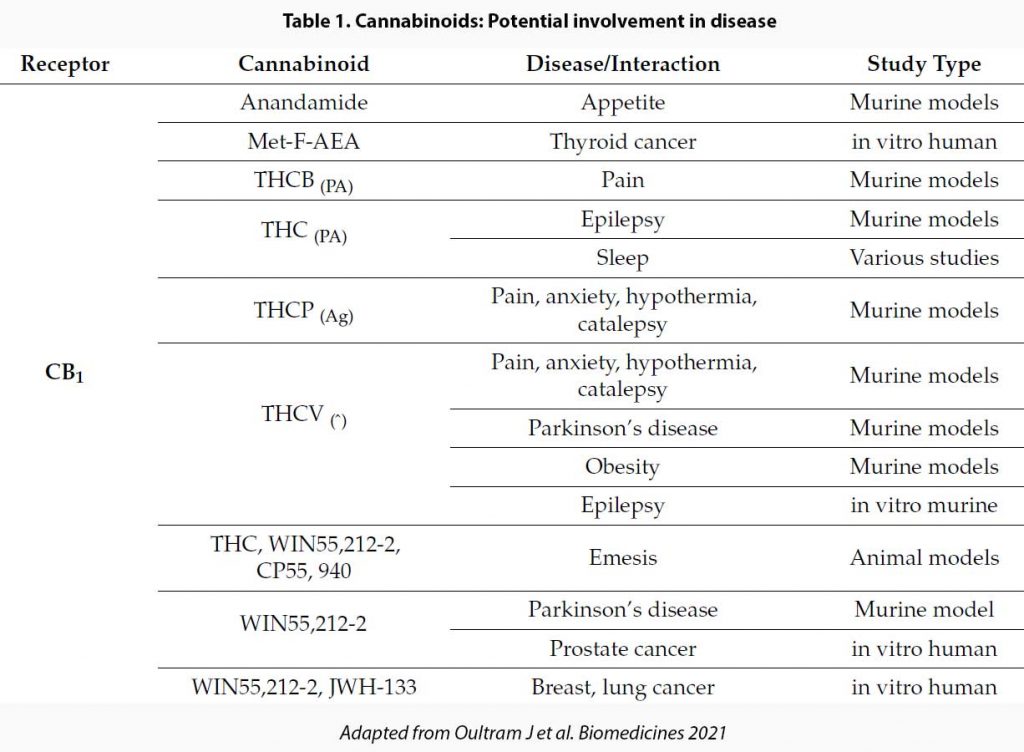
As you can see, the range of actions of CBD is pretty wide and also not necessarily predictable based on their action on the wide range of receptors. Further, actions of CBD derived from purified cannabis extracts differ from synthetic CBD because when solvents are added to extract CBD, not all impurities are removed and some THC and other cannabinoids remain. It has been suggested that these impurities may add synergistic and complementary effects. Pretty much most OTC and medicinal cannabis do not contain pure CBD.
Evidence?
CBD has been assessed in randomised controlled clinical trials in:
- Rare childhood epilepsies (1-3)
- Schizophrenia (4)
- Type 2 diabetes (5)
- Fatty liver disease (6)
- Crohn’s disease (7)
- Parkinson’s disease (8)
- Huntington’s disease (9)
- Multiple sclerosis (10)
- Cannabis dependence
Most of the clinical trials with positive findings have studied the effects of CBD at doses considerably higher than in OTC preparations (>300 mg/day or 10 mg/kg/day). At least eight randomised placebo controlled clinical trials have examined the effects of CBD at low doses. Most of these studies were small and reported limited efficacy with few adverse effects. An exception was the CAMS trial in multiple sclerosis, which examined the effects of a CBD predominant CBME, as well as a THC-based medicine on spasticity and urinary symptoms in 630 patients (10).
In a sub study of lower urinary tract symptoms (CAMS-LUTS) the CBME arm achieved a 25% reduction in episodes of urge incontinence compared to placebo (p = 0.005), although there were no changes in urodynamic studies (77). There are numerous other clinical trials done exploring the role of CBD in various diseases. See Table 2.
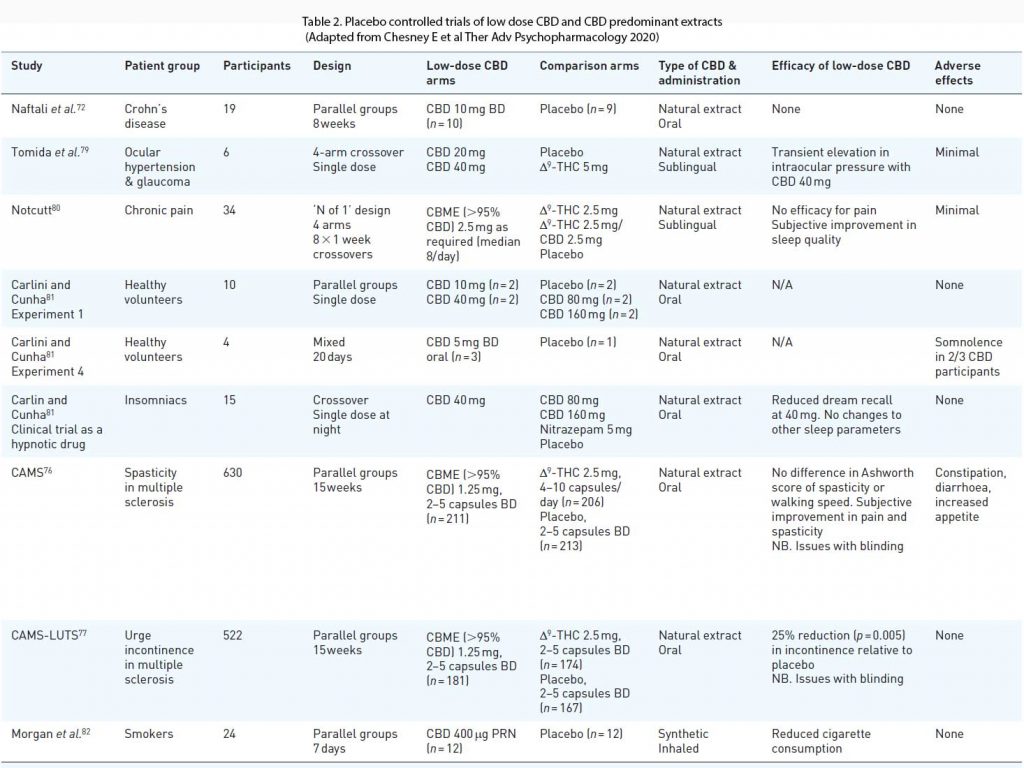
It is unknown if over-the-counter products can produce plasma concentrations of cannabidiol
high enough to have significant pharmacological effects. There is little evidence that over-the-counter cannabidiol products have therapeutic benefits. Labelling of the constituents of over-the-counter cannabidiol products may be inaccurate.
The only licensed medicine in Australia that contains CBD is the oro-mucosal spray nabiximols (Sativex), which contains similar quantities of THC and CBD, and is indicated as treatment, for symptom improvement in patients with moderate to severe spasticity due to multiple sclerosis (MS) who have not responded adequately to other anti-spasticity medication and who demonstrate clinically significant improvement in spasticity related symptoms during an initial trial of therapy. It is a Schedule 8 medication.
The Therapeutic Goods Administration (TGA) regulates the supply of medicinal cannabis. Doctors can apply to the TGA to supply medicinal cannabis to certain patients through the Authorised Prescriber Scheme and the Special Access Scheme. There is no predetermined list of conditions for which a cannabis medicine can be prescribed. Each application to prescribe is considered on its merits and assessed on a case-by-case basis. When making an application to the Australian Government’s Therapeutic Goods Administration (TGA) to prescribe a cannabis medicine, a doctor must provide clinical evidence about use of the product to enable an assessment of potential benefits and harms. It is expected that all registered conventional medicines or non-medicinal treatments will have already been explored.
Currently, there are NSW Government-funded trials investigating the safety and efficacy of cannabis medicines in:
- reducing seizures in children with severe treatment-resistant epilepsy,
- preventing nausea and vomiting in chemotherapy patients,
- enhancing the appetite and appetite-related symptoms of palliative care patients with advanced cancer,
- improving the control of symptoms, including pain, nausea and lack of appetite, in advanced cancer patients, and
- reducing chronic pain in patients with spinal cord injury.
References
- Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med 2017; 376: 2011–2020.
- Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N Engl J Med 2018; 378: 1888–1897.
- Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox–Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018; 391: 1085–1096.
- McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry 2018; 175: 225–231.
- Jadoon KA, Ratcliffe SH, Barrett DA, et al. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care 2016; 39:1777–1786.
- GW Research Ltd. Study to assess the effect of cannabidiol on liver fat levels in subjects with fatty liver disease. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01284634
- Naftali T, Mechulam R, Marii A, et al. Lowdose cannabidiol is safe but not effective in the treatment for Crohn’s disease, a randomized controlled trial. Dig Dis Sci 2017; 62: 1615–1620.
- Chagas MHN, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J Psychopharmacol 2014; 28: 1088–1092.
- Consroe P, Laguna J, Allender J, et al. Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol Biochem Behav 1991; 40: 701–708.
- Freeman RM, Adekanmi O, Waterfield MR, et al. The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicentre, randomised placebo-controlled trial (CAMSLUTS). Int Urogynecol J 2006; 17: 636–641.
- Chesney E, McGuire P, Freeman TP, Strang J, Englund A. Lack of evidence for the effectiveness or safety of over-the-counter cannabidiol products. Ther Adv Psychopharmacol. 2020 Sep 9;10:2045125320954992. doi: 10.1177/2045125320954992. PMID: 32973998; PMCID: PMC7491225.
- Oultram JMJ, Pegler JL, Bowser TA, Ney LJ, Eamens AL, Grof CPL. Cannabis sativa: Interdisciplinary Strategies and Avenues for Medical and Commercial Progression Outside of CBD and THC. Biomedicines. 2021 Feb 26;9(3):234. doi: 10.3390/biomedicines9030234. PMID: 33652704.
