9th April 2021, Dr Chee L Khoo
We are all aware of the treatment inertia in the management of patients with diabetes. In patients with heart failure, several drug classes have demonstrated significant but independent survival benefit. Quadruple therapy with an angiotensin receptor–neprilsyin inhibitor (ARNI), evidence-based β-blocker, mineralo-corticoid receptor antagonist (MRA) and sodium glucose cotransporter 2 inhibitor (SGLT2i) can cumulatively reduce the risk of death by 73% over 2 years (1). Many eligible patients with heart failure (HF) are not receiving these therapies shown to extend survival or receive them with much delay (2,3). Why is there no disquiet amongst us about the treatment inertia in patients with heart failure?
Diabetes treatment inertia
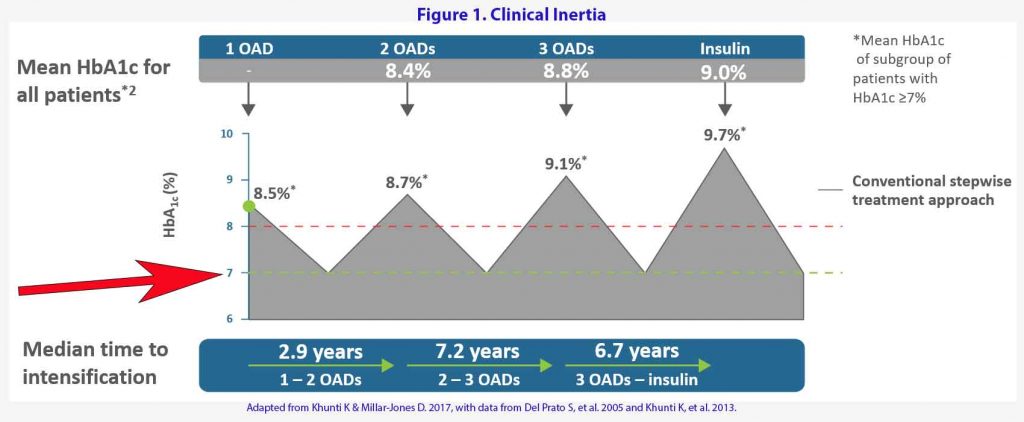
As you can see in Figure 1, we treat to failure in diabetes (4). We say we aim for a glycaemiac target of <7% but we only escalate our treatment when the patient breaches their targets PLUS many months of treatment delay. By then, the target have moved further away. We only play catch up. Over a period of 8-10 years, the patient’s glycaemic control is usually above target. This inertia or treatment delay increases mortality and morbidity (5).
What’s different with patients with heart failure?
Heart failure mortality
Heart failure (HF) is a common and frequently morbid condition with high short-term mortality (>30% one year mortality) (6). In the old days, HF is defined by reduction in ejection fraction (EF) but when we talk about HF these days, we have to differentiate between HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF). There is now HF with mid range ejection fraction (HFmrEF) which is something in between the HFrEF and HFpEF. While heart failure refers to the clinical syndrome resulting in dyspnea, exercise intolerance, or fluid retention, a reduced left ventricular ejection fraction is the sine qua non of HFrEF. Because HFrEF may be the ultimate result of dozens of heterogenous diseases, it is remarkable that over the past three decades, a consistent body of evidence has shown the effectiveness of several pharmacological therapies in improving quality of life and preventing death.
The pharmacological mainstay of established HFrEF therapy has, until recently, been a three-horse race with renin–angiotensin system (RAS) inhibitors, beta-blockers, and mineralocorticoid antagonists (MRA). The RAS inhibitors have now been replaced by the combined angiotensin receptor-neprilysin inhibitor sacubitril-valsartan (ARNI). The updated three-horse race is now a four-horse race with the addition of the SGLT2 inhibitors.
Thus, quadruple therapy consists of:
- ARNI
- Betablocker
- MRA and
- SGLT2 inhibitor.
Which agent does what?
ACEI/ARBs
- ACEI: ACE inhibitors have been shown in large RCTs to reduce morbidity and mortality in patients with HFrEF with mild, moderate, or severe symptoms of HF, with or without coronary artery disease. Data suggest that there are no differences among available ACE inhibitors in their effects on symptoms or survival.
- ARBs: ARBs have been shown to reduce mortality and HF hospitalizations in patients with HFrEF in large RCTs. Long-term therapy with ARBs in patients with HFrEF produces hemodynamic, neurohormonal, and clinical effects consistent with those expected after interference with the renin-angiotensin system. Unlike ACE inhibitors, ARBs do not inhibit kininase and are associated with a much lower incidence of cough and angioedema, although kininase inhibition by ACE inhibitors may produce beneficial vasodilatory effects.
Beta Blockers:
- Bisoprolol: All-cause mortality was significantly lower with bisoprolol than on placebo deaths with a hazard ratio of 0.66. There were significantly fewer sudden deaths among patients on bisoprolol than in those on placebo with a hazard ratio of 0.56 independent of severity of heart failure. (7)
- Carvedilol: In patients with severe heart failure, compared with placebo, carvedilol reduce the risk of death by 35% and reduce combined risk of death or hospitalisation by 24%. (8)
- Metoprolol XR: Metoprolol CR/XL once daily in addition to optimum standard therapy reduce all cause mortality by 34%. (9)
ARNI
As compared with enalapril, ARNI reduced the risk death by 16% and hospitalisation for heart failure by 21% and decreased the symptoms and physical limitations of heart failure. (10)
MRA
- Spironolactone: Blockade of aldosterone receptors by spironolactone, in addition to standard therapy, substantially reduces the risk of both morbidity and death among patients with severe heart failure by 30%. (11)
- Eplenerone: The addition of eplerenone to optimal medical therapy reduces morbidity and mortality among patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure. (12)
SGLT2 inhibitors
In EMPA-REG OUTCOME, empagliflozin showed a significantly reduced combined cardiovascular endpoint in high cardiovascular risk patients with T2DM taking empagliflozin, including a remarkable 38% relative risk reduction in all-cause cardiovascular mortality and 35% relative risk reduction in heart failure hospitalization (13)
In DECLARE-TIMI 58, patients with established cardiovascular disease or cardiovascular risk factors (including 10% with prevalent heart failure of any type) treated with dapagliflozin had no difference in rates of cardiovascular death, myocardial infarction, or ischemic stroke (14). Yet, dapagliflozin-treated patients did have a 17% reduction in cardiovascular death or heart failure hospitalization, a finding driven by a 27% relative reduction in the risk of hospitalization for heart failure.
DAPA-HF found marked reductions in cardiovascular mortality and heart failure hospitalization, with a 26% absolute risk reduction for the combined endpoint and a 17% reduction in all-cause mortality. Dapagliflozin was equally efficacious in patients with and without T2DM with respect to the prevention of cardiovascular death and heart failure hospitalization (15).
In EMPEROR-REDUCED, empagliflozin showed a 25% reduction in combined cardiovascular death or heart failure hospitalization and an 8% reduction in all-cause mortality, although the latter was not statistically significant. Like dapagliflozin in DAPA-HF, the beneficial effect of empagliflozin with respect to the primary outcome was independent of diabetes status.
Just like we see in patients with T2D, in heart failure, we also play catch up. We escalate treatment when patient ends up in hospital with more heart failure. All the 4 disease-modifying drug classes have incremental benefits i.e. each drug reduce risk of death and hospitalisation independent of
background therapy. Further, randomised control trials have demonstrated that addition of more drugs to background therapy for heart failure have not reduced the benefit of the background therapy.
There is enough evidence now that quadruple therapy for heart failure should be standard for all patients with heart failure. Many of us still see patients returning from hospital on 1-2 agents for their heart failure. This is particularly pertinent as many of our patients with T2D have heart failure. It is not just SGLT2 inhibitors that they need to be on to assist with their heart failure.
In the US, among patients with HFrEF who are eligible for therapy, 1 in 3 receive no β-blocker and 2 in 3 receive no MRA. Only 14%are prescribed an ARNI (2). Further, should patients who have heart failure but do not have diabetes be on an SGLT2 inhibitor on a private script?
Are we treating to failure in patients with heart failure? Who is responsible to escalate treatment in patients with heart failure? Is it us, the GP, the cardiologist or the endocrinologist?
References:
- Bassi NS, Ziaeian B, Yancy CW, Fonarow GC. Association of optimal implementation of sodium-glucose cotransporter 2 inhibitor therapy with outcome for patients with heart failure. JAMA Cardiol. 2020. doi:10.1001/jamacardio.2020.0898
- Metra, M, Teerlink, JR. Heart failure. Lancet Lond Engl 2017; 390: 1981–1995
- Jhund, PS, Macintyre, K, Simpson, CR, et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation 2009; 119: 515–523
- Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: A focused literature review. Prim Care Diabetes. 2017 Feb;11(1):3-12. doi: 10.1016/j.pcd.2016.09.003. Epub 2016 Oct 7. PMID: 27727005.
- Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015 Aug 7;14:100. doi: 10.1186/s12933-015-0260-x. PMID: 26249018; PMCID: PMC4528846.
- Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011 Oct 19;306(15):1669-78. doi: 10.1001/jama.2011.1474. PMID: 22009099; PMCID: PMC3688069
- Authors CI. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13
- Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–8
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001–7
- McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17
- Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357.
- Petrie MC, Verma S, Docherty KF, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA 2020; 323: 1353–1368.
- Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–1424.
