21st April 2022, Dr Chee L Khoo
We have discussed the two-way relationship between pancreatic cancer and diabetes. Patients with type 2 diabetes are at 1.5-2.0 times higher risk of developing pancreatic ductal adenocarcinoma (PDAC). In patients with T2D, the development of PDAC destabilises glucose homeostasis pretty much in all patients with T2D. In patients that do not have T2D, the development of PDAC pushes them across the threshold of T2D up to 6-12 months before the diagnosis. Thus, new onset T2D is a good biomarker for the diagnosis of PDAC with the glucose deteriorating up to 36 months before the diagnosis of T2D. But that is a very long time to be watching for rising glucose. In light of the high burden of type 2 diabetes [1] and the progressive nature of the disease [2], screening all patients with deteriorating diabetes for the rare occurrence of pancreatic cancer is not justified. Are there any other clues that can help us narrow down that search for early PDAC?
Mueller A et al. recently conducted a study to compare the HbA1c and body weight profiles of pancreatic cancer patients with diabetes deterioration in the >0.5 – 3 years before pancreatic cancer diagnosis with the corresponding profiles of non-cancer patients with deterioration in T2D (3). The data came from UK-based Clinical Practice Research Datalink (CPRD) GOLD which holds records on 1.5 million patients.
They identified all patients with T2D diagnosed with pancreatic cancer between 2004 and 2017. Patients with other cancers diagnosed before pancreatic cancer, diagnosis of diabetes diagnosed under 30 years old (possible T1D), gestational diabetes or steroid induced diabetes diagnosed before pancreatic cancer diagnosis and patients who has less than 2 HbA1c recorded in the 0.5 – 3 years prior to pancreatic cancer diagnosis were excluded.
For comparison, they identified patients with T2D but no pancreatic cancer diagnosis. The same exclusion criteria were used in the non-cancer patients. For each patient, they identified the presence of deterioration of diabetes control in the 0.5 – 3 years to the diagnosis of pancreatic cancer (the index date).
What is considered “deterioration of diabetes control”?
They considered patients to have diabetes deterioration if their baseline glucose-lowering treatment was intensified. An initial prescription for an anti-diabetic drug class was followed by 1 repeat prescription within 6 months is considered to be part of the baseline glucose-lowering treatment. Intensification of diabetes treatment is when there was a prescription for an anti-diabetic drug class that was not a baseline medication. If the initial prescription was not followed by another prescription within 6 months, then it was considered a treatment switch or reduction of therapy.
Amongst other things, they retrieved all HbA1c and body weight managements recorded in the 10 years before the index date of cancer diagnosis. They plotted the trajectories of mean and absolute HbA1c and body weight at 6-month intervals before the index date and before and after treatment intensification for each patient group.
What did they find?
There was a total of 540 pancreatic cancer patients with pre-existing type 2 diabetes and 219,627 non-cancer patients with type 2 diabetes. Among them, 237 (44%) cancer patients and 76,864 (35%) non-cancer patients had deterioration of diabetes control in the >0.5 – 3 years before the index date (date of pancreatic cancer diagnosis).
In both groups, pancreatic cancer patients were older than non-cancer patients (deterioration group: 72.3 vs. 67.2 years), and more likely to have had diabetes for more than 5 years before the index date (83% vs. 69%) and to take anti-diabetic medication at baseline (72% vs. 48%).
Not unexpectedly because T2D is a progressive disease, in the time leading up to the point of treatment intensification, mean HbA1c rose sharply in both patient groups. However, the mean increase in HbA1c was distinctly greater in pancreatic cancer patients than in non-cancer patients. Immediately after intensification of diabetes treatment, mean HbA1c declined in both patient groups, but returned to the pre-deterioration level only in non-cancer patients (See Figure 1).
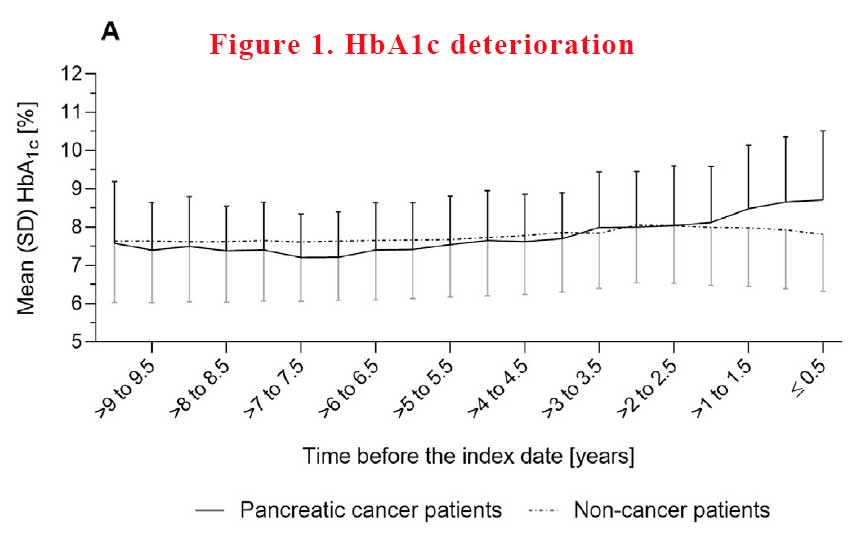
In absolute terms, HbA1c increased, on average, by 1.5% ± 1.6% in pancreatic cancer patients vs. 0.9% ± 1.4% in non-cancer patients. Increases in HbA1c of >2.0% were more frequent in pancreatic cancer patients than in non-cancer patients, with 30% vs. 16% having such pronounced changes respectively.
What happened with intensification of treatment?
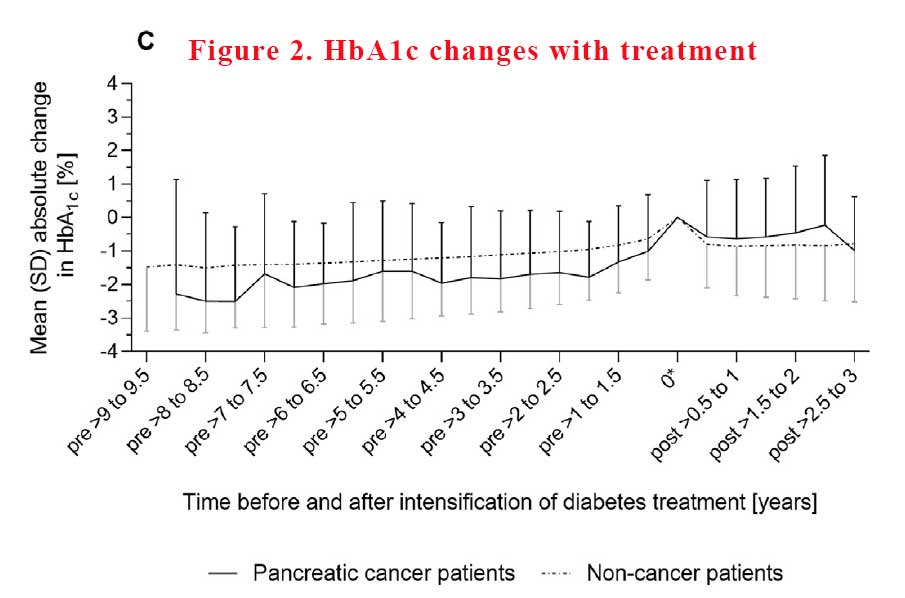
The mean absolute difference in HbA1c between the pre-deterioration period and post-intensification period, i.e. the 6-month interval after treatment intensification, was 0.9% ± 1.8% in pancreatic cancer patients compared with 0.1% ± 1.5% in noncancer patients. Differences in HbA1c of 1.0% – 1.9% between the pre-deterioration and post-intensification period were almost twice as frequent in pancreatic cancer patients as in noncancer patients (23% vs. 13%), and HbA1c differences of > 2.0% were three times more frequent (26% vs. 8%). It seems that getting control of HbA1c was harder to achieve in pancreatic cancer patients with more variability (see Figure 2).
Body weight changes
In pancreatic cancer patients, mean body weight declined continuously in the time before the intensification of diabetes treatment as well as thereafter, until cancer detection. In contrast, mean body weight remained virtually unchanged before and after diabetes treatment intensification in non-cancer patients. From the pre-deterioration period to the time of intensification of diabetes treatment, body weight decreased, on average, by 1.9% ± 6.4% in pancreatic cancer patients, whereas in non-cancer patients, body weight increased, on average, by 0.3% ± 5.2% (see Figure 3).
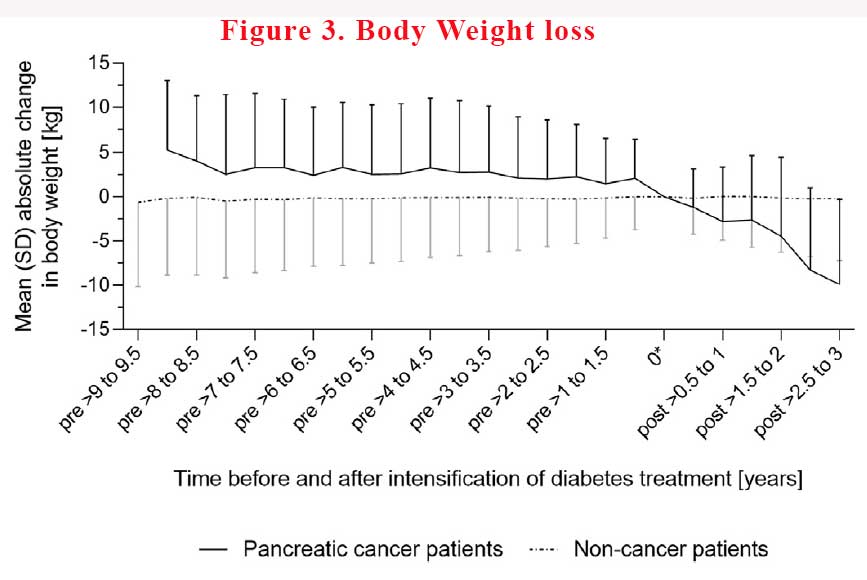
Pancreatic cancer patients had less pronounced decreases in body weight the longer treatment intensification preceded the cancer diagnosis. On average, body weight decreased by 3.6%, 2.3%, 6%, and 0.9% in cancer patients with diabetes treatment intensification in the >0.5 – 1, >1 – 2 and >2 – 3 years before the index date, respectively.
In non-cancer patients, body weight change did not depend on the timing of treatment intensification before the index date. Weight loss remain more common in pancreatic cancer patients than in non-cancer patients. However, this finding did not apply to patients who were on diet or had an insulin-based regimen at baseline. In other words, if you lose weight when you not intending to lose weight, it should raise alarm bells.
In summary, patients with T2D who developed pancreatic cancer are likely to be older (mean age 72.3 years), have diabetes for > 5 years and more likely to be on medications already. While glucose control in most patients with T2D do deteriorate over time, patients with pancreatic cancer have larger increases in HbA1c (>1.5% absolute HbA1c) up to 1-2 years before the diagnosis of pancreatic cancer. Attempts to reign in the glucose appears to be harder in patients with pancreatic cancer with persistent elevation of HbA1c despite intensification of treatment.
Body weight loss is an even more significant signal in patients with T2D who develop pancreatic cancer. There is continual weight loss from up to 1-2 years before the diagnosis of pancreatic cancer. Weight loss of >3.0% from >1 – 2 years before treatment intensification to the time of intensification was more common in pancreatic cancer patients than in non-cancer patients.
While screening for pancreatic cancer in all older patients with T2D with deteriorating glucose control and weight loss is not practical, a high index of suspicion and close monitoring of these patients is warranted.
References:
1. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 diabetes – global burden of disease and forecasted trends. J Epidemiol Glob Health 2020;10(1):107e11.
2. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009;32(Suppl 2):S151e6. Suppl 2.
3. Mueller AM, Meier CR, Jick SS, Schneider C. Characterization of the deterioration of diabetes control in patients with a subsequent diagnosis of pancreatic cancer: A descriptive study. Pancreatology. 2022 Apr;22(3):387-395. doi: 10.1016/j.pan.2022.03.012. Epub 2022 Mar 14. PMID: 35314354.
