12th May 2022, Dr Chee L Khoo
It’s been more than 4 years since we herald the arrival of the stable formulation of the new glucagon, dasiglucagon in a ready-to-use delivery device for the management of hypoglycaemia. If you think about how much time is wasted in the emergency treatment of hypoglycaemia. The existing glucagon emergency kits we currently have need reconstitution before administration. Even as a healthcare professional, it takes more than a few minutes to get ready. For the inexperienced caregiver, it definitely takes more time than that. Crucial minutes are lost when you have a patient unconscious in front of you. It’s taken 3-4 years for the dasiglucagon auto-injector to be fianally approved by the US FDA.
Fear of hypoglycaemia among individuals with diabetes is widespread, and may have a significant and negative impact on diabetes management, glycaemic control, and subsequent health outcomes (1). Further, caregivers of people with diabetes may feel concerned about administering glucagon during an emergency for fear of harming the patient (2,3).
Dasiglucagon is a novel, stable glucagon analogue in a ready-to-use aqueous solution with in-use stability data for at least 7 days at body temperature. While dasiglucagon is more stable, it still needs to prove its efficacy and safety. Does it work as well and as quickly as the old glucagon?
Efficacy
In a randomised controlled trial, 17 patients received four single subcutaneous doses (0.03, 0.08, 0.2 and 0.6 mg) of 4 mg/mL dasiglucagon under euglycaemic (plasma glucose (PG) 5.6 mmol/L) or hypoglycaemic (PG 3.1-3.7 mmol/L) conditions (4). In comparison, three doses of the commercially available glucagon (Lilly) (0.03, 0.08 and 0.2 mg) were also tested in euglycaemic conditions.
Dasiglucagon resulted a dose-dependent and rapid increase in PG levels across all doses tested. The mean increases 30 minutes post-dosing was 2.2 to 4.4 mmol/L from euglycaemia and 1.3 to 5.2 mmol/L from hypoglycaemia), which was higher than the rises elicited by similar doses of commercial glucagon which increased by 1.7-3.9 mmol/L.
When 45 participants with type 1 diabetes were randomized 3:1 to receive a single subcutaneous dose of dasiglucagon 0.6 mg or placebo following controlled induction of hypoglycaemia, dasiglucagon provided rapid reversal of hypoglycaemia (5). Plasma glucose recovery was achieved within 15 minutes by 88% of participants receiving dasiglucagon versus none receiving placebo (P < .01). The results were similar whether the injection was given into the deltoid or buttocks.
In another randomized, double-blind trial, 170 adult participants with type 1 diabetes were randomly assigned to receive a single subcutaneous dose of 0.6 mg dasiglucagon, placebo or 1 mg reconstituted glucagon in a 2:1:1 randomisation during controlled insulin-induced hypoglycaemia (6). Dasiglucagon provided rapid and effective reversal of hypoglycaemia in adults with type 1 diabetes, with safety and tolerability similar to those reported for reconstituted glucagon injection.
Rapidity of administration
How does dasiglucagon used in an autoinjector perform in real life? In an open label, randomised, crossover, comparative device handling study, trained caregivers and untrained bystanders administered the dasiglucagon autoinjector or Eli Lilly glucagon emergency kit (GEK) to manikins in a simulated emergency hypoglycaemia situation.
54 participants (18 patient-caregiver pairs and 18 bystanders) were randomised. Overall, 94% of trained caregivers were able to administer the dasiglucagon autoinjector successfully within 15 min, compared with 56% for the GEK (P < 0.05). A greater proportion of trained caregivers and untrained bystanders successfully prepared and administered the dasiglucagon autoinjector within 2 min compared with the GEK (P < 0.005 and P < 0.05, respectively). Time to successful completion was also significantly faster with the dasiglucagon autoinjector than with the GEK (P < 0.005 for both groups). Most study participants preferred the dasiglucagon autoinjector over the GEK (94%, P < 0.001) and rated it as easier (90%, P < 0.001) and less stressful to use (94%, P < 0.001) than the GEK.
The differences in the speed of administration between the two devices are notable considering the importance of rapid administration of rescue therapy in an emergency situation to limit the risk of neurologic sequelae. Severe hypoglycaemic events in patients with diabetes are associated with frequent emergency services utilisation and considerable health care cost.
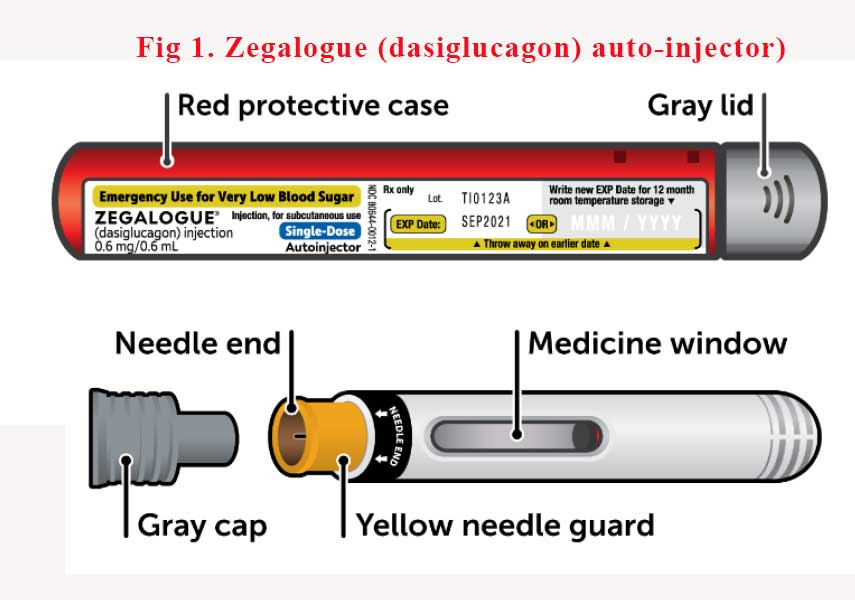
Dasiglucagon in an auto-injector device (Zegalogue®) is yet to hit Australian shores but it is only a matter of time. (See Fig 1). It’s been more than 4 years since FDA approved the device in the USA. In the meantime, please do not forget to teach carers how to reconstitute the current glucagon emergency kits.
References:
1. Wild D, von Maltzahn R, Brohan E, et al.: A critical review of the literature on fear of hypoglycemia in diabetes: implications for diabetes management and patient education. Patient Educ Couns 2007;68:10–15
2. Valentine V, Newswanger B, Prestrelski S, et al.: Human factors usability and validation studies of a glucagon autoinjector in a simulated severe hypoglycemia rescue situation. Diabetes Technol Ther 2019;21:522–530
3. Kedia N: Treatment of severe diabetic hypoglycemia with glucagon: an underutilized therapeutic approach. Diabetes Metab Syndr Obes 2011;4:337–346.
4. Hövelmann U, Olsen MB, Mouritzen U, Lamers D, Kronshage B, Heise T. Low doses of dasiglucagon consistently increase plasma glucose levels from hypoglycaemia and euglycaemia in people with type 1 diabetes mellitus. Diabetes Obes Metab. 2019 Mar;21(3):601-610. doi: 10.1111/dom.13562.
5. Bailey TS, Willard J, Klaff LJ, Yager Stone J, Melgaard A, Tehranchi R. Dasiglucagon, a next-generation glucagon analogue, for treatment of severe hypoglycaemia via an autoinjector device: Results of a phase 3, randomized, double-blind trial. Diabetes Obes Metab. 2021 Oct;23(10):2329-2335. doi: 10.1111/dom.14475.
6. Pieber TR, Aronson R, Hövelmann U, Willard J, Plum-Mörschel L, Knudsen KM, Bandak B, Tehranchi R. Dasiglucagon-A Next-Generation Glucagon Analog for Rapid and Effective Treatment of Severe Hypoglycemia: Results of Phase 3 Randomized Double-Blind Clinical Trial. Diabetes Care. 2021 Jun 1;44(6):1361-1367. doi: 10.2337/dc20-2995.
