28th August 2022, Dr Chee L Khoo
We know that low density lipoprotein cholesterol (LDL-C) is a risk factor for atherosclerotic cardiovascular disease (ASCVD). We also know that reducing LDL-C levels can significantly reduce those risks irrespective of whether they have established cardiovascular disease or not (1). That means reducing LDL-C works in secondary as well as primary prevention. However, even when LDL-C is well-controlled in statin-treated ASCVD patients, there is still a residual risk of cardiovascular disease. Perhaps, we are missing something in addition to reducing LDL-C levels. Lipoprotein(a) is an under-recognised but significant genetic risk factor for ASCVD and aortic valve disease. It is shown to be causal from data from prospective epidemiologic studies, Mendelian randomisation, and genome wide association studies [1]. Is that the missing link in the residual CV risk?
What is lipoprotein (a)?
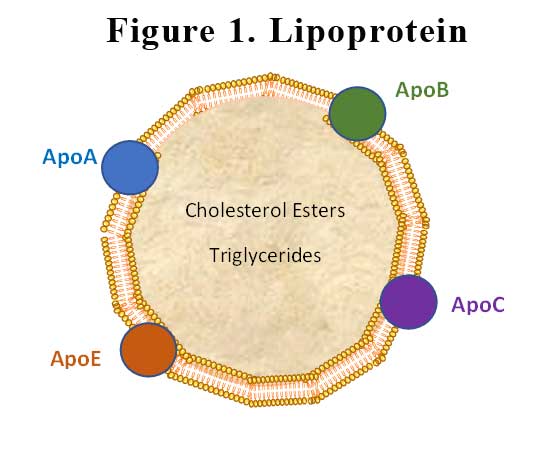
Lipoproteins have a central hydrophobic core of non-polar lipids, primarily cholesteryl esters and triglycerides. This hydrophobic core is surrounded by a hydrophilic membrane consisting of phospholipids, free cholesterol, and apolipoproteins. Plasma lipoproteins are divided into seven classes based on size, lipid composition, and the type of apolipoproteins. The apolipoprotein in lipoprotein(a) (Lp(a)) is apolipoprotein(a) (apoA), which is bound covalently to another apolipoprotein, apoB, contained in the outer shell of the particle. See Figure 1.
Lp(a) biosynthesis is the major determinant of plasma Lp(a) concentrations, though important knowledge gaps persist concerning Lp(a) production and especially, catabolism.
Is Lp(a) associated with CVD?
The evidence-base for the potential causality of elevated Lp(a) for CVD and calcific aortic valve disease (CAVD) is primarily based on studies in subjects without prior CVD or on statin therapy. A consistent conclusion is that elevated Lp(a) levels predict higher CVD event rates, both in primary care populations [2,3], as well as in subjects treated with statins [4] or PCSK9 inhibitors [5,6]. See Figure 2.
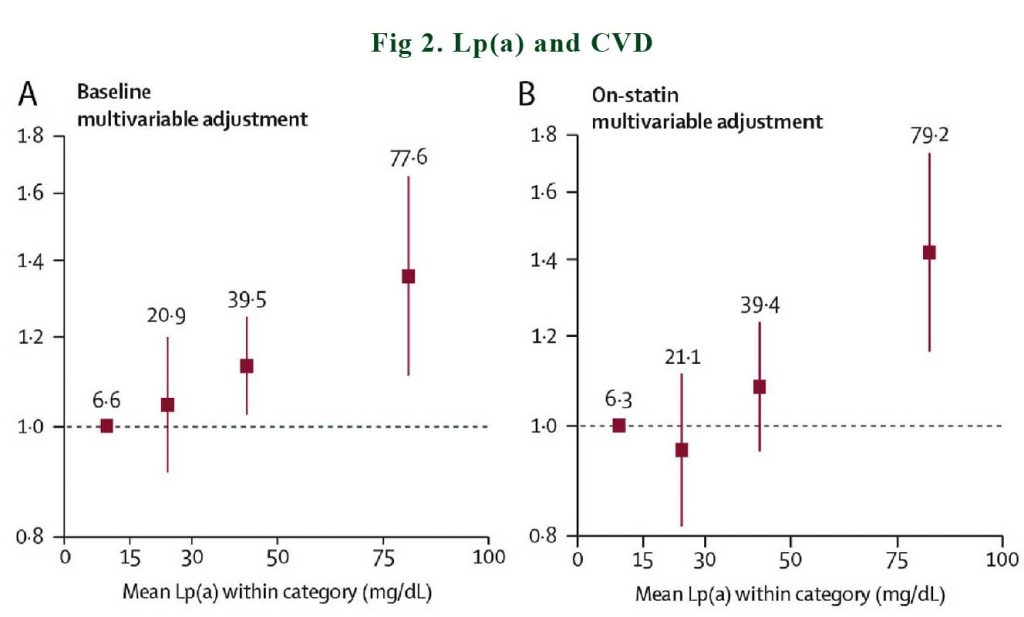
In a meta-analysis of 29,069 patients in the Lipoprotein(a) Studies Collaboration, it was documented that baseline and on-statin Lp(a) showed an approximately linear relationship with incident cardiovascular disease [4]. Similarly, Willeit et al. [4] showed lipoprotein(a) predict future ASCVD event more strongly in those on statin treatment as compared to on placebo.
In addition to its contribution to ASCVD risk, high Lp(a) drives calcification of the aortic valve, leading to aortic valve stenosis. Lp(a) is thought to be primarily important for the initiation phase of aortic valve stenosis [7].
How prevalent is elevated Lp(a)?
In the general population, it is estimated that 20% of individuals worldwide has an Lp(a) level above 50 mg/dl or 105 nmol/l. A total of 5% of individuals has an Lp(a) level above 120 mg/dl or 50 nmol/l, whereas 1% of individuals has an extremely elevated Lp(a) level above the 99th percentile, which corresponds to approximately 180 mg/dl [8]. Plasma levels of Lp(a) are also dependent on ethnicity: individuals from African descent generally have higher Lp(a) levels, whereas plasma Lp(a) levels are generally lower in Asian individuals, when compared with Caucasian individuals [9].
Can we anything about elevated Lp(a)?
Observational studies on apheresis performed specifically for elevated Lp(a) in subjects with recurrent events or progressive CVD have demonstrated 70–90% lower CV-event rates following initiation of apheresis [10-13].
In the ODYSSEY OUTCOMES trial, a 1mg/dL reduction in Lp(a) with alirocumab, a PCSK9 inhibitor was associated with a hazard ratio of cardiovascular events of 0.994 (6). That may not sound much but what if we can achieve much greater reduction of Lp(a)? Imagine what the hazard ratio would be. Indeed, an antisense oligonucleotide, Pelacarsen has just completed its Phase 2 trial. It was able to achieve 80mg/dL reduction in Lp(a) (14). Phase 3 trial is yet to recruit.
Olpasiran is a small interfering RNA targeting LPA messenger RNA which is being investigated in phase 2 and has already shown dose-dependent Lp(a) reductions up to 90% (15).
What is the definition of “high” Lp(a)?
This is not as easy as it sounds. Before we can answer that question, we need to consider how LDL-C levels are measured. Well, actually, as you know, LDL-C is not measured but is estimated from the Friedewald formula:
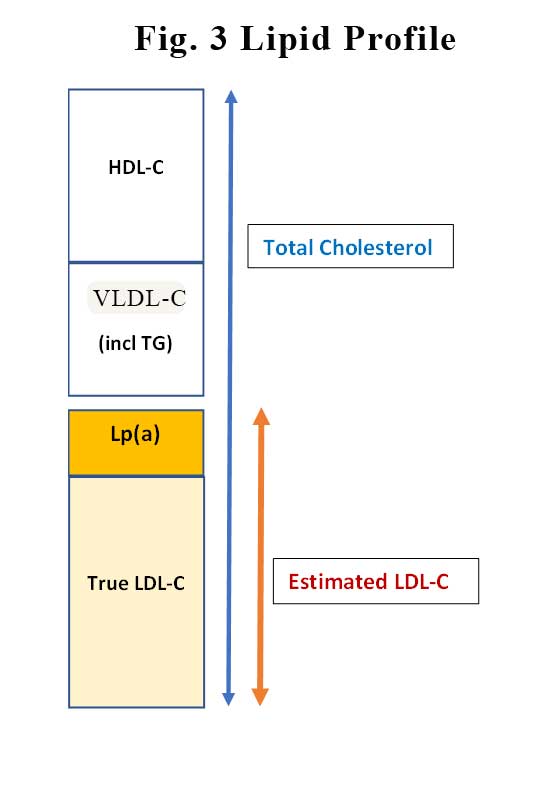
LDL-C (mmol/L) = total cholesterol – HDL-C – (triglycerides/2.2),
Essentially, LDL-C is what is left behind after you remove HDL-C and VLDL (which contains your TG) from total cholesterol. In amongst the “estimated” LDL-C that is your Lp(a). See Figure 3. So, the LDL-C estimation is less reliable when the TG is high. Similarly, as Lp(a) levels increases, the true LDL-C decreases. This might explain why the linear relationship between LP(a) and incident CVD is stronger in patients on statins. In patients on statins the LDL-attributable risk is reduced with statin treatment, Lp(a)-associated risk becomes an even stronger predictor of residual risk.
The European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) the American Society For Apheresis, the American Society for American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, the Canadian Cardiovascular Society guidelines], the National Lipid Association guidelines and the HEART UK consensus statement on Lipoprotein (a) all have different Lp(a) risk thresholds (and different recommendations on who needs Lp(a) testing). In general, increased risk is suggested to occur > 75 – 125 nmol/L. Most recommend measuring Lp(a) in individuals at moderate or higher risk, family history of CVD, recurrent events, and non-responsive to medical therapy. Most recently, the EAS/ESC guidelines recommended that all individuals should have Lp(a) measured at least once (16) in their life time.
What does this mean for your patients in primary care?
Although the main focus of CVD prevention in the lipid space has been on lowering LDL-C, elevated Lp(a) has emerged the last decade as a genetic, independent and likely causal risk factor for CVD and calcific aortic valve disease (CAVD). There are a number of Lp(a) lowering agents in the pipeline but in the meantime, measuring Lp(a) in every patient at least once to ascertain the CV risk status of the patient is probably a good idea.
It’s not just for the sake of knowing and waiting till the wonder drugs and their studies become available. Discovery of elevated Lp(a) may mean these patients may no longer have low CV risk. It may mean you have to be more aggressive in your LDL-C lowering. It may mean more closely monitoring of the aortic valve by the cardiologist. It may mean aspirin for “primary” prevention providing the bleeding risk is evaluated. It may mean perhaps, ordering a coronary artery calcium score to further ascertain the cardiovascular risk. I know I will be checking mine at the next blood test.
References:
- Nelson Wang, Jordan Fulcher, Nishan Abeysuriya, et al Intensive LDL cholesterol-lowering treatment beyond current recommendations for the prevention of major vascular events: a systematic review and meta-analysis of randomised trials including 327 037 participants, The Lancet Diabetes & Endocrinology, Vol 8, Iss 1, 2020, Pages 36-49, https://doi.org/10.1016/S2213-8587(19)30388-2
- S. Erqou, S. Kaptoge, P.L. Perry, et al. Emerging Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality, J. Am. Med. Assoc. 302 (2009) 412–423.
- P.R. Kamstrup, M. Benn, A. Tybjaerg-Hansen, et al., Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study, Circulation 117 (2008) 176–184.
- P. Willeit, P.M. Ridker, P.J. Nestel, et al., Baseline and on-statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials, Lancet 392 (2018) 1311–1320.
- M.L. O’Donoghue, S. Fazio, R.P. Giugliano, et al., Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk, Circulation 139 (2019) 1483–1492.
- V.A. Bittner, M. Szarek, P.E. Aylward, et al., Effect of alirocumab on lipoprotein(a) and cardiovascular
- Pawade TA, Newby DE, Dweck MR. Calcification in aortic stenosis: the skeleton key. J Am Coll Cardiol 2015; 66:561–577.
- Tsimikas S, Stroes ESG. The dedicated ‘Lp(a) clinic’: a concept whose time has arrived? Atherosclerosis 2020; 300:1–9.
- Pare´ G, C¸ aku A, McQueen M, et al. Lipoprotein(a) levels and the risk of myocardial infarction among 7 ethnic groups. Circulation 2019; 139:1472–1482.
- B.R. Jaeger, Y. Richter, D. Nagel, et al., Longitudinal cohort study on the effectiveness of lipid apheresis treatment to reduce high lipoprotein(a) levels and prevent major adverse coronary events, Nat. Clin. Pract. Cardiovasc. Med. 6 (2009) 229–239.
- J. Leebmann, E. Roeseler, U. Julius, et al., Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study, Circulation 128 (2013) 2567–2576.
- E. Roeseler, U. Julius, F. Heigl, et al., Lipoprotein apheresis for lipoprotein(a)-associated cardiovascular disease: prospective 5 Years of follow-up and apolipoprotein(a) characterization, Arterioscler. Thromb. Vasc. Biol. 36 (2016) 2019–2027.
- P.M. Moriarty, J.V. Gray, L.K. Gorby, Lipoprotein apheresis for lipoprotein(a) and cardiovascular disease, J. Clin. Lipidol. 13 (2019) 894–900.
- S. Tsimikas, E. Karwatowska-Prokopczuk, I. Gouni-Berthold, et al., Lipoprotein(a) reduction in persons with cardiovascular disease, N. Engl. J. Med. 382 (2020) 244–255.
- Koren MJ, Moriarty PM, Neutel J, et al. Abstract 13951: safety, tolerability and efficacy of single-dose Amg 890, a novel sirna targeting Lp(a), in healthy subjects and subjects with elevated Lp(a). Circulation 2020; 142:A13951; https://doi.org/10.1161/circ.142.suppl_3.13951.
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias:l modification to reduce cardiovascular risk. Eur Heart J 2020; 41:111–188.
