8th January 2025, A/Prof Chee L Khoo

Obstructive sleep apnoea (OSA) often (but not always) lead to daytime sleepiness and suboptimal daytime performance (including driving performance). It can also have a major effect on the quality of life (QOL) of the patient and the family. OSA is associated with a number important metabolic and cardiovascular conditions in a multidirectional manner. The traditional continuous positive airway pressure (CPAP) treatment is considered gold standard therapy but is CPAP treatment enough as the management of OSA? How efficacious is CPAP as a treatment option? Besides, what does CPAP treatment achieve? Is there any other better treatment option?
Pathophysiology of OSA
OSA is the most common sleep disorder in adults that is evaluated at sleep centres. Upper airway crowding from physical narrowing as well as collapse of the soft tissue structures in the pharynx that induce airway obstruction. Increasing values of BMI progressively worsen upper airway collapsibility [1]. Abnormalities in pharyngeal muscle innervation and oedema of the soft tissues can also affect the stability of those pharyngeal structures.
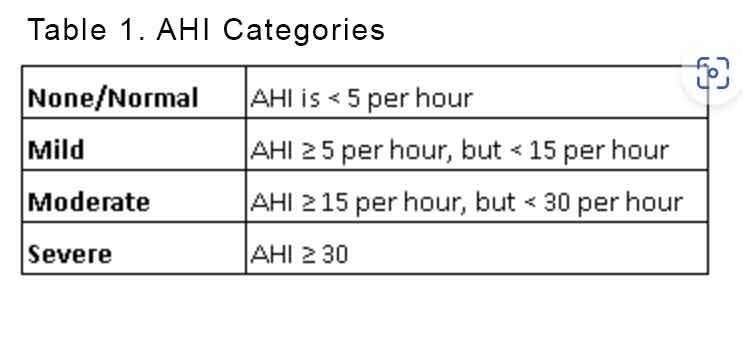
Impaired ventilation is often quantified by the apnoea, hypopnoea index (AHI) which not only determine whether the patient has OSA but also categorise the severity of the OSA. See Table 1.
Prevalence of OSA
The likelihood of a higher apnoea-hypopnoea index (AHI), a measure of OSA severity, is 14 % greater per each point increase in body mass index (BMI) and 61 % higher per each 0.1 unit increase in waist/hip ratio [2]. In addition, a 10 % weight gain predicts a 32 % increase in AHI and a 6 times risk of developing moderate to severe OSA, while a 10 % weight loss predicts a 26 % drop in AHI [3].
Multi-directional relationship between OSA and its co-morbidities
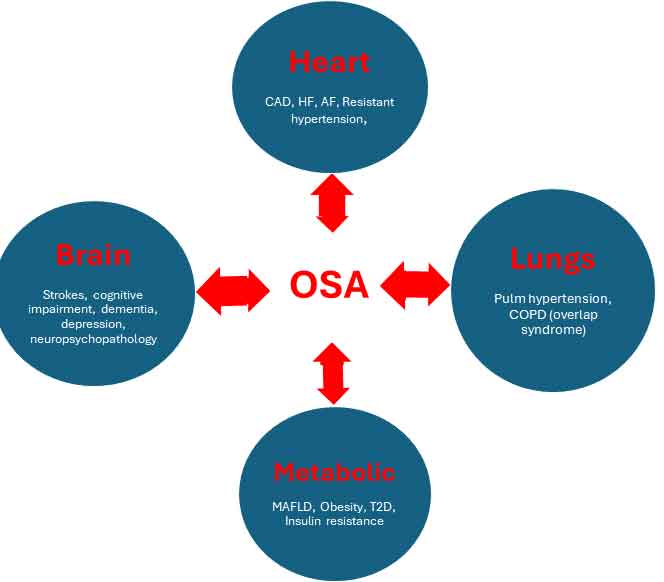
OSA is more than just daytime impairment. The body’s response to the hypoxia leads to fluctuating intrathoracic pressure and recurring micro-arousals, which generate cell and molecular consequences that include sympathetic excitation, systemic inflammation and oxidative stress, in addition to metabolic and endothelial dysfunction [4–7].
It is then not surprising that OSA is associated with obesity, hypertension (especially resistant hypertension), atrial fibrillation, heart failure, coronary artery disease, cerebrovascular disease, pulmonary hypertension, dementia, insulin resistance, depression and COPD (overlap syndrome) in a multi-directional manner.
See Figure 1.
Diagnostic testing for OSA
There are four types of sleep monitoring devices (I–IV). Type I is the traditional, standard polysomnography (PSG) which is an overnight test conducted when one is asleep in a sleep lab with a sleep technologist in attendance. PSG uses ≥7 channels to monitor sleep, respiration, and muscle activity.
Types II–IV are portable sleep monitoring or home sleep apnoea test (HSAT). Type II is like type I except there is no sleep trained personnel in attendance while Type III HSAT uses four to seven channels and type IV uses one to two channels with at least one being a pulse oximeter.
HSAT is recommended for the evaluation of patients with high clinical probability of OSA along with a comprehensive sleep evaluation. Home-based type III and IV type sleep studies should be used to “rule-in” but not “rule-out” moderate to severe OSA.
Which screening questionnaires?
Screening questionnaire can assess for daytime sleepiness and/or assess the probability of OSA based on presence of physical risk factors. The Epworth Sleepiness Scale is a self-administered questionnaire designed to assess a person’s level of daytime sleepiness. It correlates poorly with the presence of OSA. The BERLIN and STOP-BANG scores have 8-10 questions looking at both daytime sleepiness and probability of OSA. The NoSAS have 5 questions assessing the risk of OSA. The OSA50 only has 4 questions assessing the same.
Management of OSA
Different types of PAP
We not only have CPAP devices but all sorts of other positive airway pressure (PAP) devices. Bilevel PAP (BPAP) delivers different inspiratory and expiratory pressures are capable of reducing the average pressure required to keep the airway open in patients with OSA and could conceivably reduce some CPAP-related side effects. There are also auto-titrating PAP (machine auto-titrated pressure) as well as adaptive servo-ventilation (adjustable bilevel positive pressure support with a backup rate which is used to treat central sleep apnoea syndromes), all hoping to improve adherence by reducing the discomfort of PAP devices.
There are also different types of mask interfaces apart from the traditional tight nasal masks.
“Efficacy” of PAP
CPAP is the gold standard OSA treatment. Many studies have demonstrated the efficacy of symptom improvement in patients with symptomatic OSA. In a systematic review, Patil et al. showed that CPAP treatment resulted in clinically significant reduction in disease severity as evidenced by complete resolution or near resolution of the AHI, improvement in subjective sleepiness scores, ability to maintain wakefulness and sleep related QOL.
All well and good but that is only in patients with symptomatic OSA (e.g. based on the ESS). Patil et al. also noted that there is “insufficient and inconclusive evidence to either recommend or withhold PAP to treat non-sleepy adults with OSA as a means to reduce CV events or mortality”.
What about non-sleep related outcomes?
Theoretically, when we address the ventilation impairment and the secondary effects of hypoxia, we should reduce mortality and the other co-morbidities.
Mortality
In the same systematic review Patel et al found conflicting conclusions regarding all-cause mortality (8). Similarly, a recent multi-centre RCT from Spain (9) did not demonstrate significant benefit with CPAP therapy compared to usual care for either all-cause mortality or CV mortality in a group of non-sleepy subjects with acute coronary syndrome. It is important to note that the mean adherence to CPAP therapy in this trial was only 2.78 h/night.
Hypertension
It would seem that in patients with resistant hypertension, there is a BP reduction with CPAP therapy, in the range of 5–7 mmHg in systolic BP and 3–5 mmHg in diastolic BP (10,11). Other meta-analyses have demonstrated reductions in BP in patients on CPAP treatment for moderate to severe OSA in the magnitude of reduction is 2-4 mmHg.
Atrial fibrillation
Patients with OSA who are not treated with CPAP are significantly more likely to have recurrent AF than CPAP-treated patients, with two- to six-fold higher adjusted risk of recurrence. However, a more recent RCT involving 109 patients with paroxysmal AF and moderate to severe OSA, did not demonstrate reduction in AF burden despite CPAP used were > 4 hours per night over 5 months (12).
Heart failure
CPAP treatment of OSA in this population improves systolic and diastolic BP, heart rate, heart rate variability (HRV) and may improve left ventricular function and diastolic function although adherence to therapy is vital to see the above benefits.
Stroke
There have been mixed results about the efficacy of CPAP treatment on stroke outcomes, but currently there is promising evidence suggesting CPAP improves cognitive functioning of stroke patients, which is consistent with the benefits of CPAP in the general OSA population. Earlier diagnosis of OSA and early treatment could improve overall health and cognitive status and reduce the risk of recurrent stroke and stroke mortality.
COPD and overlap syndrome
CPAP have shown benefits in improving respiratory function in patients with COPD and improving driving performance and reducing accidents but the data is not robust.
Insulin resistance
PAP therapy has been associated with improvement in glycaemic index and insulin sensitivity but the evidence is of low quality. Comprehensive weight loss strategies including bariatric surgery and incretin therapy is worthy of consideration if conservative therapy fails.
As you can see, while CPAP treatment is efficacious in sleep improvement with daytime benefits, data on metabolic and cardiovascular benefits is not as robust. Let’s not just treat the symptoms of OSA. Let’s treat the underlying cause in the majority of cases of OSA. It’s the weight management we need to work on.
Weight management
Since upper airway crowding from physical narrowing is a major factor in the pathophysiology of OSA in a majority of (but not all) patients, weight loss must accompany any management of OSA.
Bariatric surgery is effective in reducing BMI and has an impact on reducing OSA severity although a considerable number of patients undergoing bariatric surgery continue to experience severe and moderate OSA post- surgery. Most of the data comes from older bariatric surgical techniques and we await more up-to-date data on the newer surgical techniques.
In the SCALE trial, in obese subjects with moderate to severe OSA 3mg daily liraglutide led to a 4% weight loss and a reduction of 6 events/hr (13). 50% of subjects remain with severe OSA. Only 5% achieved OSA remission. Semaglutide has not been studied in an population with obesity and OSA.
In the recent double-blind, randomised, controlled SURMOUNT-OSA trial, 469 adults with moderate to severe OSA and obesity were randomised to receive either weekly tirzepatide 15mg or placebo. Trial 1 consist of adults who are not on CPAP while trial 2 consist of adults already on CPAP. Tirzepatide reduced the AHI (by -58,7% vs -3%), body weight, hypoxic burden, hsCRP concentration and systolic blood pressure and improved sleep-related patient-reported outcomes.
Pharmacists, dentists and other specialists can refer patients for HSAT and prescribe a CPAP device to treat the sleep problems. Patients can also self-refer to industry-run clinics and then a device is prescribed. They are only treating the symptoms of OSA and not the other co-morbidities that come with OSA. Device people prescribe devices.
With tirzepatide, we now have an option to CPAP as a treatment of OSA. Currently, tirzepatide is not approved in Australia for treatment of OSA but in patients who are obese, chances are they will have OSA. Rather than treating the symptoms of OSA, tirzepatide can be used to treat the obesity and they may have benefit of treatment of the other CV and metabolic comorbidities.
Just because the patient is prescribed CPAP doesn’t mean the devices are being used. They may not be used for the whole sleeping period. They may not be used every night. The threshold most often used for “adherent” is used >4 h per night on >70% of nights over a rolling 30-day period over a defined period of time.
HOT OFF THE PRESS!!
On December 20, 2024, U.S. Food and Drug Administration approved tirzepatide for the treatment of moderate to severe obstructive sleep apnoea (OSA) in adults with obesity, to be used in combination with a reduced-calorie diet and increased physical activity.
References:
- Kirkness JP, Schwartz AR, Schneider H, Punjabi NM, Maly JJ, Laffan AM, et al. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol 2008 Jun;104(6):1618–24
- Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA 2003 May 7;289(17): 2230–7.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000 Dec 20;284 (23):3015–21
- Castro-Grattoni AL, Alvarez-Buvé R, Torres M, et al. Intermittent hypoxia-induced cardiovascular remodeling is reversed by normoxia in a mouse model of sleep apnea. Chest 2016; 149: 1400–1408.
- Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia–revisited–the bad ugly and good: implications to the heart and brain. Sleep Med Rev 2015; 20: 27–45.
- Garvey JF, Taylor CT, McNicholas WT. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J 2009; 33: 1195–1205.
- Vgontzas AN, Gaines J, Ryan S, et al. CrossTalk proposal: metabolic syndrome causes sleep apnoea. J Physiol 2016; 594: 4687–4690.
- 39. Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med 2019;15(2):335–343.
- Sanchez-de-la-Torre M, Sanchez-de-la-Torre A, Bertran S, et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med 2020;8(4):359–367.
- Iftikhar IH, Valentine CW, Bittencourt LR, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens 2014;32(12):2341–2350; discussion 2350.
- Liu L, Cao Q, Guo Z, Dai Q. Continuous positive airway pressure in patients with obstructive sleep apnea and resistant hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich) 2016;18(2):153–158.
- Traaen GM, Overland B, Aakeroy L, et al. Prevalence, risk factors, and type of sleep apnea in patients with paroxysmal atrial fibrillation. Int J Cardiol Heart Vasc 2020;26:100447.
- Blackman A, Foster GD, Zammit G, Rosenberg R, Aronne L, Wadden T, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes 2016 Aug;40(8):1310–9
- Malhotra A, Bednarik J, Chakladar S, Dunn JP, Weaver T, Grunstein R, et al. Tirzepatide for the treatment of obstructive sleep apnea: rationale, design, and sample baseline characteristics of the SURMOUNT -OSA phase 3 trial. Contemp Clin Trials 2024 Jun;141:107516
- Chang et al. International Consensus Statement on Obstructive Sleep Apnea. Int Forum Allergy Rhinol. 2023 July ; 13(7): 1061–1482.
