30th March 2025, A/Prof Chee L Khoo
While invasive coronary angiogram is still the gold standard in deciding whether revascularisation is indicated, invasive coronary angiogram has its limitations. That’s why intravascular ultrasound (IVUS) was invented. Well, it’s been 25-30 years since then and we have yet to have IVUS widely available for clinical use. We have additional miniaturised imaging techniques (see below later) since then and they are also not in clinical use as such. However, the data collected from these new imaging modalities have really revolutionised our understanding of acute coronary syndrome and its management.
Our current management target in coronary artery disease is the culprit lesion shown up in invasive angiogram. Obstructive coronary atherosclerotic plaques (those that are angiographically severe, typically defined as diameter stenosis of ≥70%) are associated with adverse cardiovascular outcomes. This was demonstrated in the Coronary Artery Surgery Study (CASS) back in 1993 (1). With conservative management, lesions with luminal diameter stenosis of ≥90% had a 50% higher rate of progression to MI than lesions with diameter stenosis of 50% (1). Remember, the first statin, lovastatin was only commercially available in 1987 and conservative management is poor by today’s standards. And the more obstructive lesions there are, the worse the outcomes were. Patients with two- vessel or three- vessel CAD had significantly higher rates of MI and death than those with one- vessel CAD or no disease (2).
While earlier (before current aggressive medical management) studies have shown coronary artery bypass graft (CABG) surgery reduced the risk of death and MI in symptomatic patients with multivessel and left main CAD as well as in those with left ventricular dysfunction (3), studies over the last few decades have shown that revascularisations vs medical treatment have similar major adverse cardiovascular events (MACE) (4-6).
We know that acute coronary syndromes (ACS) often arise from rupture of severe stenotic plaques (>70%) but we also know that ACS can arise from rupture of mildly stenotic plaques. Obviously, it is not the degree of stenosis alone that determine the likelihood of rupture. Or perhaps, the degree of stenosis as demonstrated on invasive angiogram may not be accurate. This is where data from other intravascular imaging modalities have contributed to our new knowledge about what constitute a vulnerable plaque – plaques that are prone to rupture.
You would think that invasive coronary angiogram is the gold standard but invasive coronary angiograms are 2D depictions of a 3D structure and luminal geometry is often inaccurately represented. CT coronary angiography (CTCA), on the other hand can identify features of high- risk vulnerable plaques: positive remodelling, low- attenuation plaque, spotty calcification and a napkin- ring sign (luminal narrowing by a low-attenuating eccentric structure (indicative of a necrotic core) surrounded by a thin ring- like hyperattenuating rim (a thin cap)) (7-9).
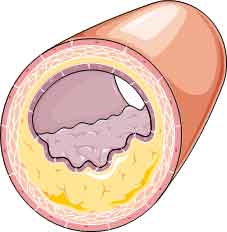
The IVUS most widely used is grey-scale IVUS, in which acoustic sound waves are reflected off a plaque and the backscatter amplitude pattern of the gathered waveform is converted into pixel- based representations of coronary tissue ranging from black (radio- lucent) to white (radio- reflective). This technology allows for visualisation of the coronary lumen, vessel and plaque (including accurate dimensional measurements) but is limited in its capacity for detailed plaque characterisation. In particular, it cannot identify thin fibrous cap or necrotic core within the plaques (see Figure 1).
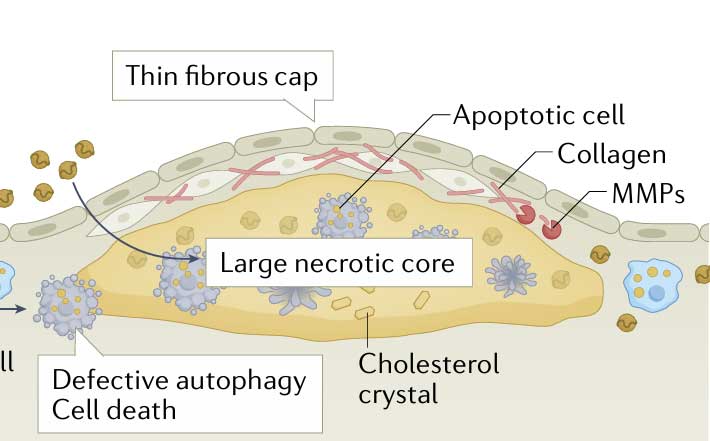
Optical coherence tomography (OCT) is an invasive coronary imaging modality in which back- scattered infrared light is analysed to determine plaque dimensions and structure. It has a tenfold higher axial resolution than that of IVUS and is, therefore, the only imaging modality that can precisely measure true fibrous cap thickness. OCT can also be used to differentiate lipid from fibrous tissue more accurately than IVUS and additionally allows for the visualisation of macrophages, microchannels, neovascularisation, plaque rupture and thrombosis.
Near- infrared spectroscopy (NIRS) can be used to detect the chemical signature of cholesterol and cholesterol esters and is the most accurate FDA- approved, catheter- based tool to identify lipids in plaques106. Compared with IVUS and OCT, NIRS identifies lipid content in plaques more accurately even in the presence of dense calcification107. NIRS is also potentially capable of identifying inflammation, cell proliferation and apoptotic cells in a fibrous cap.
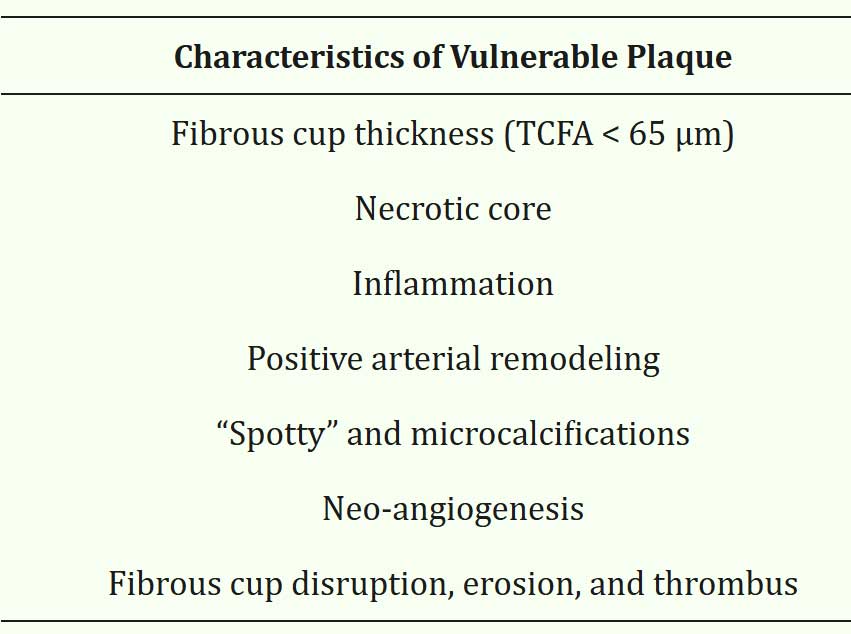
Thus, data from the advance imaging modalities have allowed us to characterise the vulnerable plaque, features that predispose a plaque to rupture and thrombosis causing ACS. See Table 1.
So, how does all this technology help you and me in our practice seeing our patients who may or may not have atherosclerosis?
Point 1
A non-obstructive CTCA does not totally reassure us. Any evidence of early atherosclerosis means there is more to come. Remember, a 30% stenosis can still rupture and cause an ACS overnight. We need to consider these patients as patients with established cardiovascular disease and treat their CV risk factors aggressively as if this is secondary prevention. See Figure 2 below.
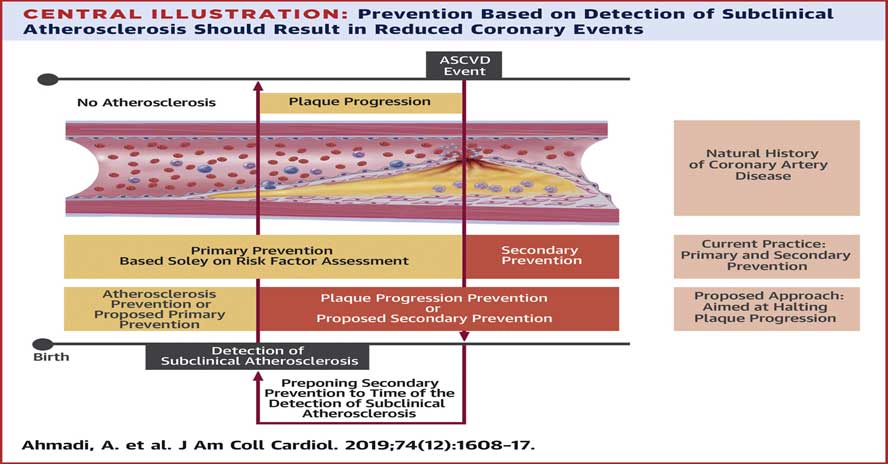
As you can see, rather than chasing after secondary prevention, we should be preventing progression and/or rupture of subclinical atherosclerosis.
Point 2
A negative functional stress test (whether stress echo or radionuclide stress testing) also doesn’t reassure us. All they say is that there is nothing >70% stenosis. Multiple non-obstructive (<70%) stenoses put these patients at super high risk of an ACS. In patients with significant CV risks may still need a CT coronary angiogram despite a negative stress test.
Point 3
Pay attention to the details of the characteristics of the plaques as described in the report. Don’t just read the summary of the report. Perhaps, a call to your friendly radiologist might be useful. Ask about evidence of any atherosclerosis however minor it may be. Ask about presence of soft or mixed plaques. Mixed plaques means it contains both soft and calcified plaques. Soft plaques are vulnerable plaques. Spotty calcifications in plaques are also vulnerable plaques.
References:
- Alderman, E. L. et al. Five- year angiographic follow- up of factors associated with progression of coronary artery disease in the Coronary Artery Surgery Study (CASS). J. Am. Coll. Cardiol. 22, 1141–1154 (1993)
- Maddox, T. M. et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 312, 1754–1763 (2014).
- Yusuf, S. et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 344, 563–570 (1994).
- Boden, W. E. et al. Optimal medical therapy with or without PCI for stable coronary disease. N. Engl. J. Med. 356, 1503–1516 (2007).
- The BARI 2D Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N. Engl. J. Med. 360, 2503–2515 (2009)
- Maron, D. J. et al. Initial invasive or conservative strategy for stable coronary disease. N. Engl. J. Med.382, 1395–1407 (2020).
- Motoyama, S. et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J. Am. Coll. Cardiol. 50, 319–326 (2007).
- Shmilovich, H. et al. Vulnerable plaque features on coronary CT angiography as markers of inducible regional myocardial hypoperfusion from severe coronary artery stenoses. Atherosclerosis 219, 588–595 (2011).
- Puchner, S. B. et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J. Am. Coll. Cardiol. 64, 684–692 (2014)
