14th July 2021, Dr Chee L Khoo

The risk for people in the general population of developing type 1 diabetes (T1D) is about 1 in 300. For those who have a family member with T1D, the risk is 1 in 20. T1D progresses over 3 stages. In stage 1, two or more autoantibodies are already slowly attacking the β-cells. This can occur years before clinical diagnosis of T1D. Plasma glucose starts to become abnormal in stage 2 but the patient is still largely asymptomatic. In stage 3, T1D symptoms are present due to significant β-cell loss. Many patients with T1D are diagnosed in dramatic fashion when they are hospitalised with diabetic ketoacidosis, a serious and potentially life-threatening condition. Once diagnosed, can we prevent further destruction of the remaining beta cells?
Preserving the function of remaining β cells, before or after diagnosis, makes subsequent management of patients with T1D a lot easier. Once diagnosed, patients often enjoy a brief honeymoon period whereby the rest of the beta cells die. A series of clinical trials have been done in recent-onset type 1 diabetes in an attempt to preserve β-cell function, primarily with immunotherapies and often targeting T cells. Some of these efforts have had modest initial success, but without robust durable effects.
In a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial, patients with recent-onset T1D (<100 days from diagnosis), aged 18–45 years, have at least one type of diabetes-associated autoantibody, and with a peak stimulated C-peptide of greater than 0·2 nmol/L were enrolled from nine medical centres in the USA (n=8) and Australia (n=1). Participants were randomly assigned (2:1) to receive either oral 400 mg imatinib mesylate or matching placebo for 26 weeks.
Treatment assignments were masked for all participants and study personnel except pharmacists at each clinical site. The primary endpoint was the difference in the area under the curve (AUC) mean for C-peptide response in the first 2 h at 12 months in the imatinib group versus the placebo group. adjusting for sex, baseline age, and baseline C-peptide, with further observation up to 24 months.
The results
43 participants in the imatinib group and 21 in the placebo group were included in the primary intention to treat analysis at 12 months. The 2-hour C-peptide area-under-the curve (AUC) mean in response to a 4-hour mixed meal tolerance test (MMTT) at 12 months was 0·583 nmol/L for the imatinib group and 0·489 nmol/L for the placebo group and constituted a 19·4% treatment effect. However, this effect was lost at 24 months.
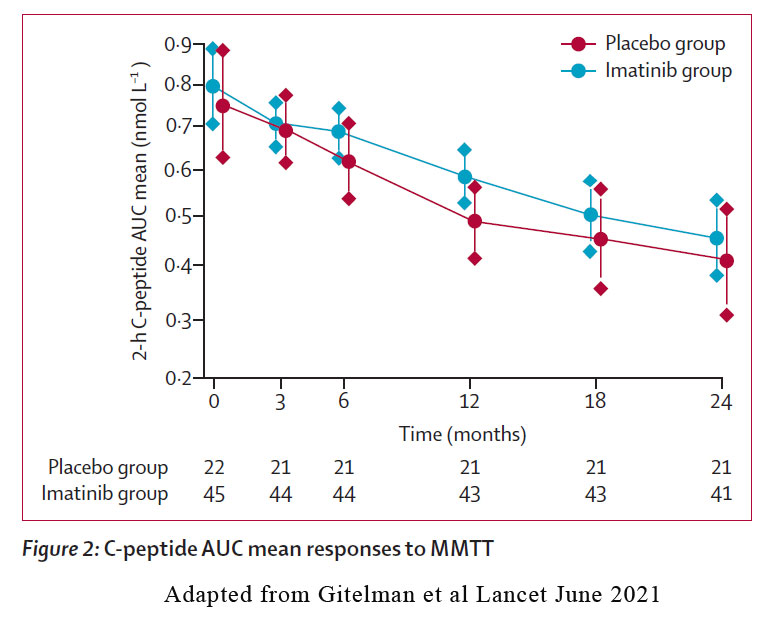
Exogenous insulin use was similar at baseline between the two groups but was used at notably lower doses in the imatinib group versus the placebo group at the first follow-up assessment at 3 months. This difference persisted at the 6-month assessment but was lost at the 12-month assessment. Short acting insulin accounted for most of the differences. HbA1c initially decreased lower than baseline levels in both groups and was lower in the imatinib group than in the placebo group during the active treatment phase, with the greatest difference at 3 months.
MMTT-stimulated responses showed distinct differences between the imatinib and placebo groups during the treatment period (figure 4, appendix p 1). 2-h glucose concentration was decreased in the imatinib group at 3 months and 6 months. Interestingly, the 2-h insulin secretion AUC was similar between the imatinib and placebo groups during the active treatment period and up to 24 months. Consequently, dose-response curves for insulin secretion and plasma glucose were flatter in the placebo group than in the imatinib group.
The slope of the dose-response function for insulin secretion and plasma glucose increased with imatinib treatment to higher than baseline level at 3 months, and stabilised up to 6 months, but decreased thereafter when participants were off active treatment. This is a measure of β-cell glucose sensitivity which increased with imatinib treatment.
Insulin secretion rates calculated at matched glycaemia (7 mmol/L) were markedly higher in the imatinib group than in the placebo group at 6 months. Adiponectin markedly increased in the imatinib group during the 6 months on therapy. Adiponectin, an adipokine secreted by adipocytes, is thought to increase insulin sensitivity by improving glucose and lipid metabolisms.
Immunological effects
No statistically significant changes in immune system B cell function in terms of autoantibody titres were noted with time in the imatinib group versus the placebo group. Immune phenotyping by flow cytometry indicated no substantial differences in B cells, T cells, or myeloid cells between the imatinib and placebo groups.
Adverse reactions
During the 24-month follow-up, 32 (71%) of 45 participants who received imatinib had a grade 2 severity or worse adverse event, compared with 13 (59%) of 22 participants who received placebo. The most common safety issues associated with imatinib use included gastrointestinal issues (primarily nausea that was self-limited and transient) and additional laboratory issues (associated with changes in liver function tests and abnormal complete blood cell counts).
What is Imatinib?
The is a first trial of a tyrosine kinase inhibitor in recent-onset type 1 diabetes, utilising the first-in-class drug member, imatinib. Imatinib is a 2-phenyl amino pyrimidine derivative that functions as a specific inhibitor of a number of tyrosine kinase enzymes. It has had notable success as a therapy for chronic myelogenous leukemia and that might also affect both immunological and metabolic pathways. It occupies the TK active site, leading to a decrease in activity. There are TK enzymes in insulin receptors.
Imatinib might act via a range of different mechanisms to alter the course of type 1 diabetes. Direct effects of imatinib on β-cell function include increased glucose-stimulated insulin secretion, and enhanced β-cell survival in the presence of various stressors, including a high-fat diet and autoimmunity.
This study showed that 26 weeks of treatment with imatinib slowed the decrease in β-cell function for up to 12 months. Although the effect was not sustained out to 24 months, this study demonstrates a novel means of improving beta cell health and insulin sensitivity. If the effect of imatinib had been mediated solely by a change in insulin resistance, rather than also on ß-cell function, then one would expect less insulin secreted for a given glucose level, rather than our observation of greater insulin secretion, as compared to findings in the placebo group.
This study also further our understanding on how to slow down beta cell demise in patients with T1D as well as in patients with T2D. Interesting times.
***. TrialNet is recruiting in south west Sydney targeting relatives of patients with T1D. Screening is offered at no cost to eligible individuals to evaluate their personal risk of developing the disease. This unique screening can identify the early stages of type 1 diabetes (T1D) years before any symptoms appear. It also helps researchers learn more about how T1D develops and plan new studies exploring ways to prevent it. Contact me if you have interested patients or their relatives.
References:
Gitelman SE, Bundy BN, Ferrannini E, Lim N, Blanchfield JL, DiMeglio LA, Felner EI, Gaglia JL, Gottlieb PA, Long SA, Mari A, Mirmira RG, Raskin P, Sanda S, Tsalikian E, Wentworth JM, Willi SM, Krischer JP, Bluestone JA; Gleevec Trial Study Group. Imatinib therapy for patients with recent-onset type 1 diabetes: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2021 Jun 29:S2213-8587(21)00139-X. doi: 10.1016/S2213-8587(21)00139-X. Epub ahead of print. PMID: 34214479.
