13th September 2021, Dr Chee L Khoo
Phase 3 trials have inherent limitations in assessing vaccine safety because of the small number of participants and the sample population is generally reasonably young and healthy. They are often underpowered to identify less common adverse events. For example, the AZ interventional arm had about 11,000 participants and the interventional arm of the Pfizer Comirnaty vaccine had about 23,000 participants. Post-marketing surveillance is required to monitor the safety of new vaccines in real-world settings.
Not withstanding that everything to do with Covid-19 is rapidly changing, trying to collect information about adverse events related to vaccination is not easy. Passive surveillance systems such as the Vaccine Adverse Event Reporting System (VAERS) 6 collect information about adverse events that are potentially related to vaccination. This information is voluntarily reported by health care providers and the public. These systems are useful for quickly identifying potential safety signals, which, compliments the findings of phase 3 trials.
Active surveillance systems such as the Biologics Effectiveness and Safety (BEST) system (9) aim to compare the incidence of adverse events of interest in large electronic health record databases with the background historical incidence. Although active surveillance can help highlight suspicious trends, the lack of a rigorously constructed comparable control group limits the ability of such surveillance to identify causal effects of vaccination.
Adverse events from mRNA vaccines
As of May 24, 2021, nearly 5 million people in Israel, comprising more than 55% of the population, had received two doses of the Pfizer Comirnaty (BNT162b2) vaccine (1). The integrated data repositories of the largest health care organisation in Israel were used to evaluate the safety profile of the BNT162b2 vaccine (2). They compared the incidence of a broad set of potential short- and medium-term adverse events among vaccinated persons with the incidence among matched unvaccinated persons. Mild adverse events such as fever, malaise, and local injection-site reactions were not included in this study. The study included 42 days of follow-up, which provided 21 days of follow-up after each of the first and second vaccine doses.
Results
There were a range of adverse events thought to be associated with vaccination but a number of events stood out. Vaccination was most strongly associated with an elevated risk of myocarditis (risk ratio: 3.24). The increased risk of myocarditis translated to approximately 3 excess events per 100,000 persons. Among the 21 persons with myocarditis in the vaccinated group, the median age was 25 years (interquartile range, 20 to 34), and 90.9% were male.
Lymphadenopathy, appendicitis and herpes zoster infection were also increased with vaccination (risk ratio: 2.43, 1.40 and 1.43 respectively. See Table 1.
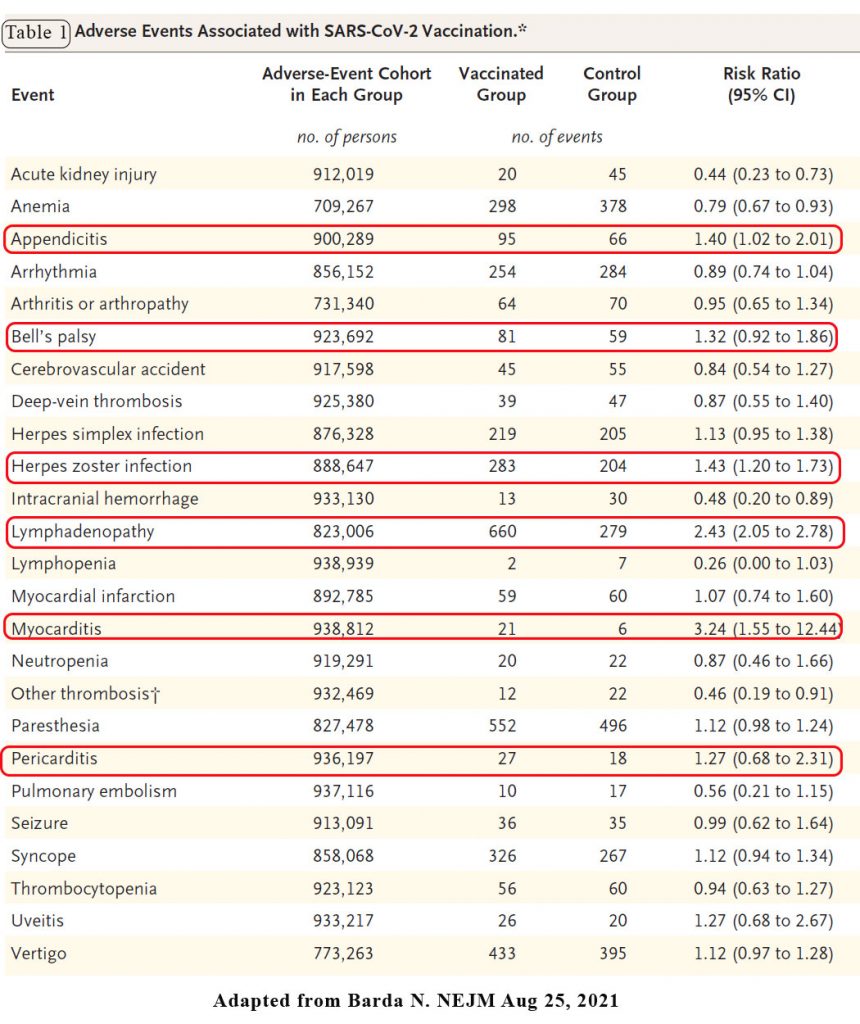
Another vaccine-related adverse event that has recently received attention in the medical literature is Bell’s palsy. The effect estimate was consistent with a potentially mild increase in the risk of Bell’s palsy after vaccination, with a risk ratio of 1.32. The absolute effect was small, with up to 8 excess events per 100,000 persons.
As of 26 June 2021, a total of 322 million doses of vaccine were used in the US, and thus far, 79 children aged 16 or 17 years, and 196 young adults aged 18–24 years have been confirmed by the CDC as having myocarditis or pericarditis following mRNA COVID-19 vaccination (Pfizer and Moderna) after analysing data in the VAERS [3]. The adjusted risk ratio for myocarditis and pericarditis events in children and young adults between 16 and 24 years of age has been determined to be 0.94 (4,).
The European Medicine Agency’s safety committee (PRAC) has included 145 cases of myocarditis in the European Economic Area (EEA) among people who received Comirnaty and 19 cases following the use of (Moderna) Spikevax. As of 31 May 2021, around 177 million doses of Comirnaty and 20 million doses of Spikevax had been given in the EEA. As of end of May 2021, the incidence of myocarditis is 1 per million for both Comirnaty and Spikevax in the EEA.
Clinical features
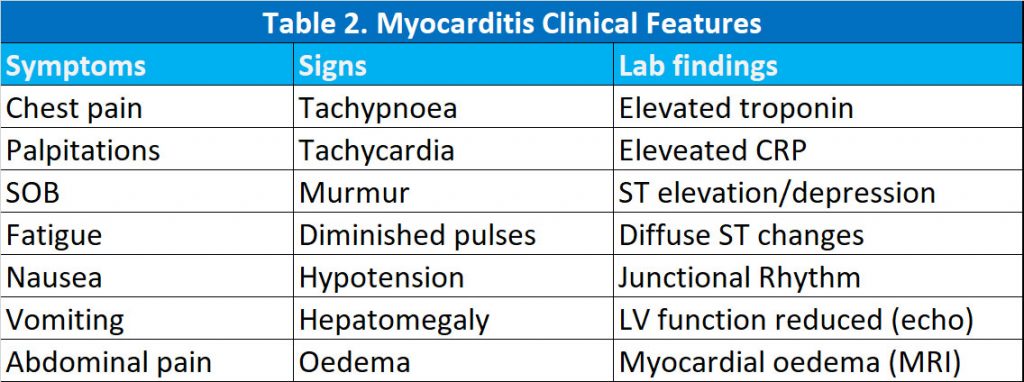
In a review of 29 published cases of myocarditis/pericarditis, Das et al, described their clinical characteristics (6). 13 patients were < 18 years. All patients were male. Three (10%) cases were after the first dose of vaccine and the rest were after the second dose of the vaccine. The common clinical presentation of COVID-19 vaccine-associated myopericarditis includes chest pain, fever, palpitation, shortness of breath, fatigue, nausea, vomiting, abdominal pain or unusual symptoms such as forceful or thumping heart beats. The onset of symptoms occurred most commonly within 3 days but ranged between 1 and 7 days. Common signs of myopericarditis include tachypnoea, tachycardia, murmur, gallop, diminished pulses, hypotension, hepatomegaly, oedema and signs of low cardiac output.
All cases where troponin and C-reactive protein (CRP) were reported had elevated levels. The most common abnormal ECG finding was ST-segment elevation in 11 (38%), followed by diffuse ST changes in 10 (35%); other changes included peaked T wave (1), junctional rhythm (1), non-specific ST changes (1), ST depression (1) and normal in (4) patients.
Echocardiography showed mild dysfunction in 13 patients, of whom most were age > 18 years. The patients who were > 30 years of age had reduced left ventricle (LV) function in 66% (4/6) cases, whereas LV function was reduced in 34% (8/23) in young adults < 30 years of age. In children < 18 years where an echocardiogram was available, the LV function was primarily normal in all patients.
Cardiac magnetic resonance imaging (CMR) was available in 20 (69%) patients, and the presence of late gadolinium enhancement (suggesting myocardial injury) was present in all reported cases. In contrast, additional evidence of myocardial oedema on T2 mapping was present only in seven (35%) patients. In most cases, patients required hospitalisation for 2–7 days, a median of only 3 days. All patients recovered and were discharged home in less than a week. Two patients aged > 18 years had non-sustained ventricular tachycardia during hospitalization. Only one patient aged > 18 years required inotropes and mechanical circulatory support.
Most patients were treated with non-steroidal anti-inflammatory drugs (NSAID) alone, followed by NSAID with colchicine and NSAID with steroids. In patients < 18 years of age where treatment was reported, four patients received intravenous immunoglobulin (IVIG) and steroids. In all cases where follow-up was available, documented complete clinical recovery in 1–3 weeks during follow-up.
Obviously, the risk of cardiac complications due to SARS-CoV-2 infection far exceeds the minimal and rare risks of vaccination-related transient myocardial or pericardial inflammation.
As of 13th September, kids between 12-16 years old can now book online to be vaccinated with the Pfizer Comirnaty vaccine. Many of these young adults would prefer their GP to vaccinate them rather than going to the vaccination hubs to be jabbed. We better be informed about some of the latest data on the potential adverse events relating to the vaccine.
References:
- Our World in Data. Coronavirus (COVID-19) vaccinations. Statistics and research (https://ourworldindata .org/covid-vaccinations#source -information-country-by-country).
- Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, Hernán MA, Lipsitch M, Kohane I, Netzer D, Reis BY, Balicer RD. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med. 2021 Aug 25:NEJMoa2110475. doi: 10.1056/NEJMoa2110475. Epub ahead of print. PMID: 34432976; PMCID: PMC8427535.
- Recent Update on mRNA Vaccine. Available online: https://www.cdc.gov/vaccines/acip/meetings/slides-2021-06.html (accessed on 12th Sept 2021).
- Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine Emergency Use Authorization; US Department of Health and Human Services, Food and Drug Administration: Silver Spring, MD, USA, 2021. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine (accessed on 12th Sept 2021).
- Vaccine and Related Biological Products Advisory Committee (VRBPAC) 10 June 2021, Meeting Presentation. Available online: https://www.fda.gov/media/150054/download (accessed on 15 June 2021)
- Das BB, Moskowitz WB, Taylor MB, Palmer A. Myocarditis and Pericarditis Following mRNA COVID-19 Vaccination: What Do We Know So Far? Children (Basel). 2021 Jul 18;8(7):607. doi: 10.3390/children8070607. PMID: 34356586; PMCID: PMC8305058.
