
10th October 2021, Dr Chee L Khoo
Updated 17th October 2021
You would have caught a bit of the announcement that some people may be due a Covid-19 vaccine booster. As usual, the announcement is not precise or detailed enough and now everyone is confused whether they are the intended target group for the booster. It is not a call to boosters for most vaccinated people. It’s only for those who has a significant reduction in their immunity. It’s not for those who has “a weakened immune system” or “a bit run down”. As usual, we are the last to be given the details of who we are targeting. The eligibility criteria is detailed below.
The rationale for the booster
Patients with immunocompromising conditions or therapies are at increased risk of severe outcomes due to COVID-19. Studies have shown that the immune-compromised population with COVID-19 have a 1.5-2.0 times higher risk of death than the general population (1-3). In addition, immunocompromised individuals can have prolonged SARS-CoV-2 infection and viral shedding, which can increase the risk of viral evolution during infection, and the subsequent risk of development of viral variantsm(4-10)
Unfortunately, people who has immunocompromising conditions or are on therapies that reduce their immunity, may not develop adequate immunity from the standard double vaccinations of the Covid-19 vaccines, whether AZ or Pfizer. The ATAGI has now announced that the following people should be getting their third vaccine:
Who is eligible?
Active Cancers
- Active haematological cancers – e.g leukaemia, lymphoma
- Non-haematological cancers currently on active treatment including chemotherapy, radiotherapy, and/or hormonal therapy
Recipients of organ transplants
- Solid organ transplant and still on immunosuppressive therapy
- Bone marrow transplant within 2 years of transplantation.
- Those beyond 2 years from transplant should discuss with their treating specialist about the need for a 3rd dose.
The immunosuppressive agents that qualify
- High dose corticosteroid treatment equivalent to >20mg/day of prednisone for ≥14 days in a month, or pulse corticosteroid therapy.
- Medications used to treat auto-immune arthritis (e.g Rheumatoid arthritis or Ankylosing Spondylitis)
- mycophenolate,
- methotrexate (>0.4 mg/kg/week),
- leflunomide,
- azathioprine (>3mg/kg/day),
- 6-mercaptopurine (>1.5 mg/kg/day),
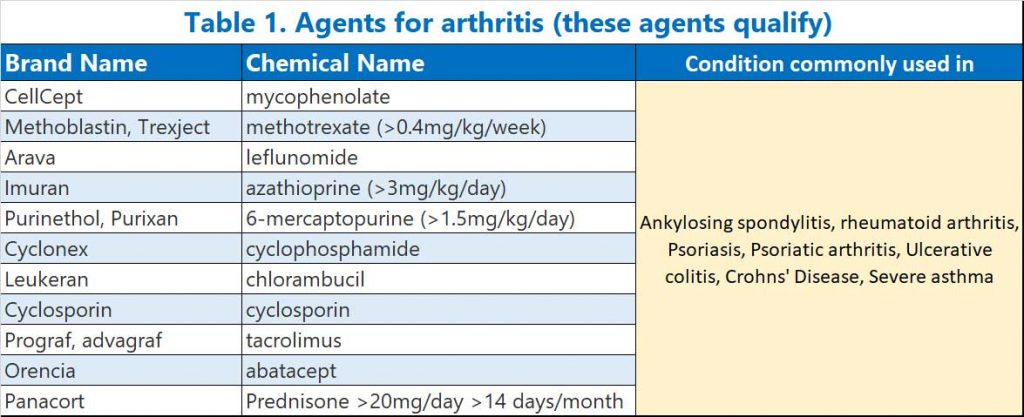
- alkylating agents (e.g. cyclophosphamide, chlorambucil), and systemic calcineurin inhibitors (e.g. cyclosporin, tacrolimus) (see Table 1)
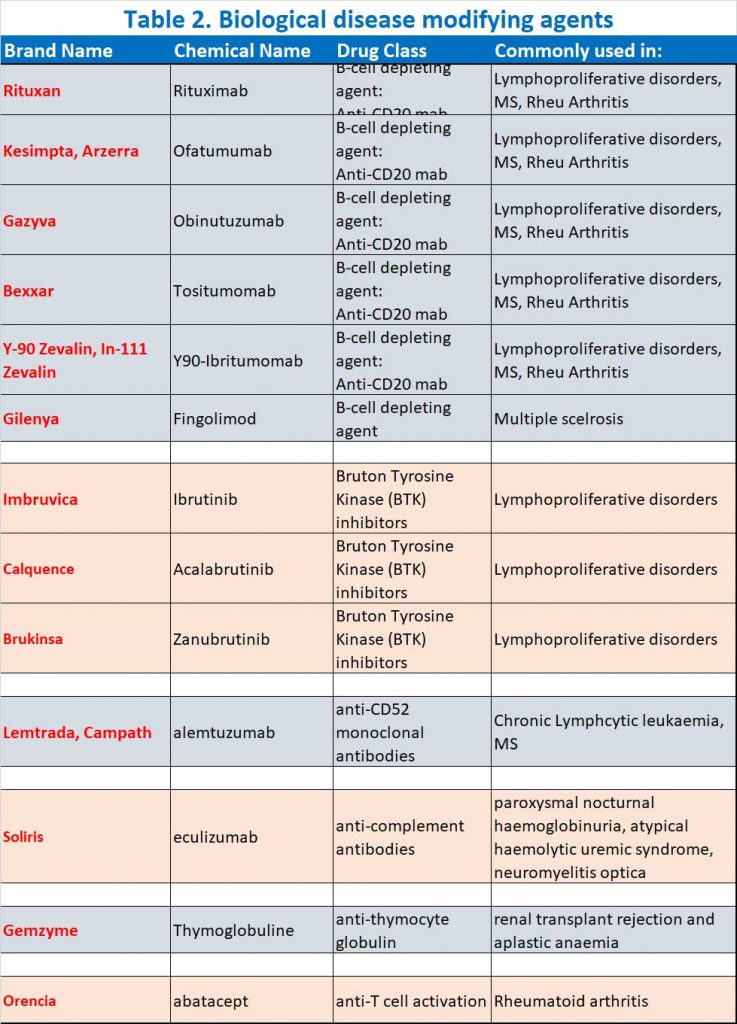
- B cell depleting agents (e.g. anti-CD20 monoclonal antibodies, BTK inhibitors, fingolimod), anti-CD52 monoclonal antibodies (alemtuzumab), anti-complement antibodies (e.g. eculizumab), anti-thymocyte globulin (ATG) and abatacept (see Table 2)
- Janus kinase inhibitors (or so-called Jak nib inhibitors) – this class was not mentioned in the announcement by ATAGI but is listed together with b-cell depleting agents, prednisone and methotrexate in the a recent study (12) which exhibit impaired SARS-CoV-2 vaccine-induced immunity (see Table 2A).

Agents that don’t qualify
These agents are also used to treat auto-immune conditions but do not significantly affect immunity and patients on these agents are not recommended to have a third injection at this point in time.
- Immune checkpoint inhibitors,
- anti-integrins,
- anti-TNF-α,
- anti-IL1, anti-IL6, anti-IL17, anti-IL4 and anti-IL23 antibodies
- hydroxychloroquine (Plaquenil) or sulfasalazine (Pyralin)
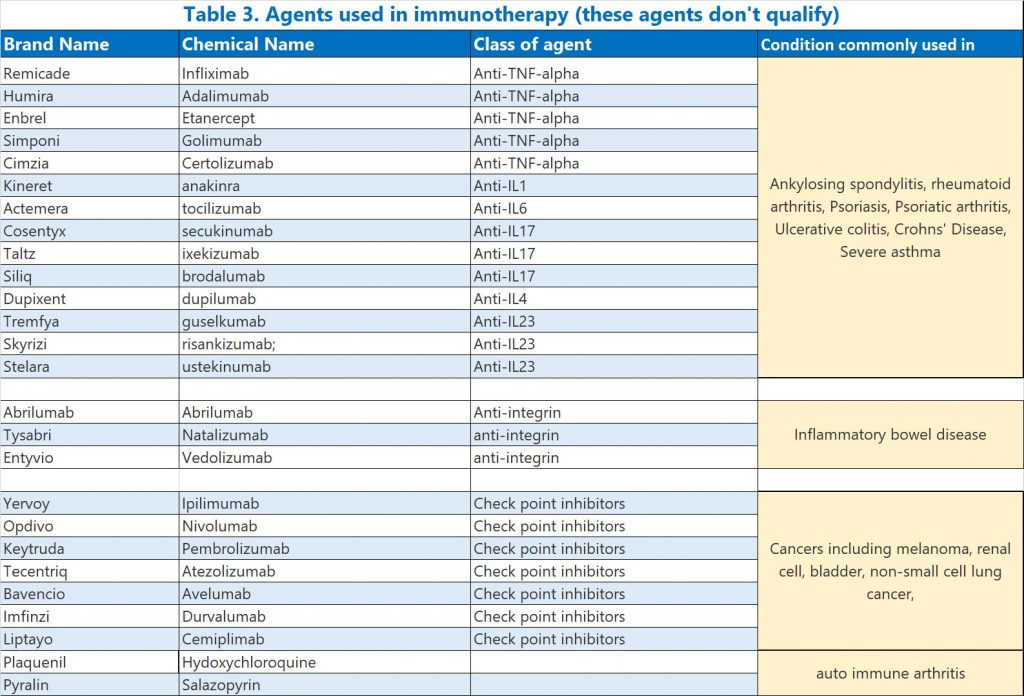
See Table 3.
While these agents on their own do not significantly reduce immunity, they may do so when used in combination. Patients who are on a combination of these agents should discuss with their GP or specialist.
Diseases that results in immunosuppression
- Primary immunodeficiency including combined immunodeficiency and syndromes, major antibody deficiency (e.g., common variable immune deficiency (CVID) or agammaglobulinemia), defects of innate immunity (including phagocytic cells), defects of immune regulation, complement deficiencies and phenocopies of primary immunodeficiencies.
- Advanced
or untreated HIV with CD4 counts <250/μL or those with a higher
CD4 count unable to be established on effective antiretroviral therapy
- a 3rd primary dose is not required for people living with HIV, receiving ART with CD4 counts ≥250/μL
- Long term haemodialysis or peritoneal dialysis
Which vaccine?
An mRNA vaccine (Pfizer or Moderna) is preferred to Vaxzevria (AstraZeneca) for this 3rd dose. AstraZeneca can be used for the 3rd dose for individuals who have received AstraZeneca for their first 2 doses if there are no contraindications or precautions for use, or if a significant adverse reaction has occurred after a previous mRNA vaccine dose which contraindicates further doses of mRNA vaccine (e.g., anaphylaxis, myocarditis).
When should you have the booster?
The recommended interval for the 3rd dose is 2 to 6 months after the 2nd dose of vaccine.
- A minimum interval of 4 weeks may be considered in exceptional circumstances (e.g., anticipated intensification of immunosuppression; outbreaks).
- People who have received a 2nd dose more than 6 months ago should receive a 3rd dose as soon as feasible.
Who else might be eligible?
Anyone with a condition not listed above should only be considered for a 3rd dose where the treating physician has assessed the patient as having a similar level of severe immunocompromise and where the benefits of a 3rd dose of COVID-19 vaccine outweigh the risks.
References:
1. Suarez-Garcia I, Perales-Fraile I, Gonzalez-Garcia A, et al. In-hospital mortality among immunosuppressed patients with COVID-19: Analysis from a national cohort in Spain. PLoS One 2021;16:e0255524.
2. Vaid N, Ardissino M, Reed TAN, et al. Clinical characteristics and outcomes of immunosuppressed patients hospitalized with COVID-19: experience from London. J Intern Med 2021;289:385-94.
3. Ward D, Gortz S, Ernst MT, et al. The effect of immunosuppressants on the prognosis of SARS-CoV-2 infection. Eur Respir J 2021.
4. Avanzato VA, Matson MJ, Seifert SN, et al. Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer. Cell 2020;183:1901- 12 e9.
5. Baang JH, Smith C, Mirabelli C, et al. Prolonged Severe Acute Respiratory Syndrome Coronavirus 2 Replication in an Immunocompromised Patient. J Infect Dis 2021;223:23-7.
6. Choi B, Choudhary MC, Regan J, et al. Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host. N Engl J Med 2020;383:2291-3.
7. Helleberg M, Niemann CU, Moestrup KS, et al. Persistent COVID-19 in an Immunocompromised Patient Temporarily Responsive to Two Courses of Remdesivir Therapy. J Infect Dis 2020;222:1103-7.
8. Khatamzas E, Rehn A, Muenchhoff M, et al. Emergence of multiple SARS-CoV-2 mutations in an immunocompromised host. medRxiv 2021:2021.01.10.20248871.
9. Nakajima Y, Ogai A, Furukawa K, et al. Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient. J Infect Chemother 2021;27:387-9.
10. Truong TT, Ryutov A, Pandey U, et al. Increased viral variants in children and young adults with impaired humoral immunity and persistent SARS-CoV-2 infection: A consecutive case series. EBioMedicine 2021;67
11. https://www.health.gov.au/sites/default/files/documents/2021/10/atagi-recommendations-on-the-use-of-a-third-primary-dose-of-covid-19-vaccine-in-individuals-who-are-severely-immunocompromised_1.pdf
12. Deepak, P, Wooseob, K, Paley, MA. et al. Glucocorticoids and B Cell Depleting Agents Substantially Impair Immunogenicity of mRNA Vaccines to SARS-CoV-2. MedRxiv. doi: https://doi.org/10.1101/2021.04.05.21254656
