13th October 2022, Dr Chee L Khoo

Obesity, type 2 diabetes mellitus (T2D), fatty liver disease, and cardiovascular disease (CVD) are complex and very inter-related cardio-metabolic conditions. Most of these conditions have lipids derangements as a major contributor to complications. While the link between lipids, in particular LDL-C, and CVD is linear and robust, there is still residual risks after aggressive treatment with lipid lowering agents. Further, because there is such a close connection between all the metabolic conditions, there may be a unifying culprit (apart from the lipid derangements) which many be causing the damage in these cardiometabolic conditions. Indeed, there is increasing evidence that ceramides might be that unifying factor. Let’s have a look at what ceramides are and the evidence linking them to cardiometabolic disorders.
Ceramide and sphingolipid metabolism
Sphingolipids are one of the major classes of lipid. Ceramides are one of the many derivatives of sphingolipids. Over the past two decades, accumulating evidence has shown a clear indication that sphingolipids, especially ceramides have important roles in the pathogenesis of both type 1 and 2 diabetes and its associated complications.
Sphingolipid metabolism
The biosynthetic pathway of sphingolipids consists of a complex network of synthetic and degradative reactions. Ceramides can be created by one of three pathways:
- De novo synthesis: condensation of serine and palmitoyl-CoA to form 3-ketosphinganine at the cytosolic surface of the ER. 3-ketosphinganine is reduced to spinganine whereby one of the many fatty acids by one six ceramide synthases (CerS1-6) into dihydroceramide. This is then metabolised into ceramides by desaturases, DES1 and DES2. Each distinct ceramide species contain a different fatty acid of different chain length (ceramides C14-C26).
- Complex sphingolipids can be broken down by the sphingolipid salvage pathway into ceramides by ceramidases (CDase)
- Ceramides can also be generated when sphingomyelin is hydrolysed by sphingomyelinase
See Figure 1
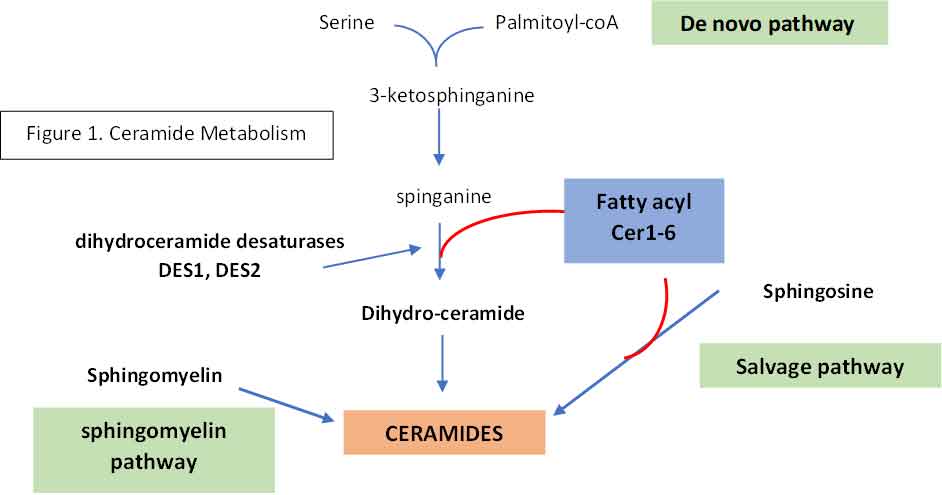
Not all ceramides are the same. Different biological functions have been attributed to different ceramides and current research aims at assigning them to individual ceramide molecular species. In this context, it has already been indicated that ceramides with specific acyl chain lengths (C16:0 and C18:0) have a metabolic impact [52]. In contrast, other ceramide molecular species (C24:0) do not, but what accounts for this specificity remains ill-defined.
Role of ceramides in disease
Apoptosis
Apoptosis of β-cells contributes significantly in the pathogenesis of both type 1 and type 2 diabetes. The role of ceramides in β-cell apoptosis in both types of diabetes is well established. There are 3 ways ceramides can effect apoptosis:
Extrinsic pathways – The signalling begins outside the cell with the binding of death ligands, such as TNF-α and Fas ligand, to their respective cell surface death receptors such as TNF receptor and Fas. This binding of the receptor by its ligand results in the recruitment and activation of initiator caspase (mostly caspase-8), which then propagates apoptosis by cleaving and activating downstream effector caspases such as caspase-3. In caspase-3 knockout mice, the absence of caspace-3 protect mice from developing diabetes [49], signifying the role of caspase cascade in β-cell apoptosis. FFA, at low glucose concentration, increased all ceramide species, but at elevated glucose concentration increased the more toxic C18:0, C22:0 and C24:1 isoforms of ceramide specifically to enhance apoptosis.
Mitochondrial membrane permeation – Ceramide induces β-cell apoptosis by increasing the mitochondrial membrane permeability, leading to the activation of the intrinsic pathway of apoptosis.
Endoplasmic reticulum (ER) stress – The ER, being the site of synthesis and folding of secreted proteins, is highly susceptible to stress when the demand for insulin folding and secretion exceeds its capacity. This increased ER stress leads to accumulation of misfolded and unfolded insulin in the ER. which may activate Unfolded Protein Response (UPR) to restore the normal ER function. When restoration fails, UPR switches in to an alternative mode and turns on the apoptotic signaling pathway in the β-cells. Fatty acids, particularly saturated FAs have been shown to promote ER stress to induce β-cell apoptosis. The mediator, once again, is ceramide.
See Figure 2.
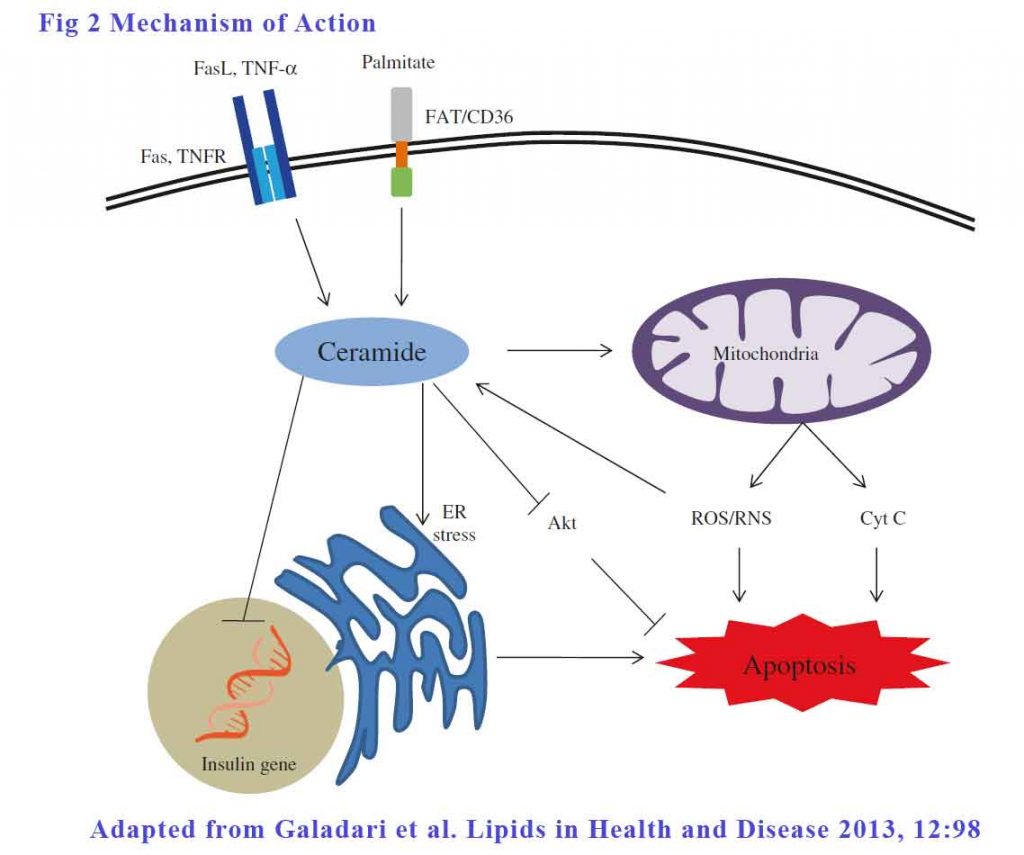
Insulin Resistance (IR)
Evidence from various studies revealed that excess FFA intake, glucocorticoid administration, obesity, and lack of physical exercise are some of the important causations leading to insulin resistance. Plethora of studies using wide variety of cultured cells, animal models and human subjects demonstrated ceramide as the key intermediate linking all these conditions to insulin resistance. Amongst the many effects of ceramide inhibits Akt which is the agent which generates GLUT4 which in turn is the membrane receptor which absorb glucose into cells.
Studies conducted using insulin-resistant human subjects demonstrated almost two-fold increase in ceramide accumulation compared to normal subjects. In another study, lipid infusion in humans is found to increase skeletal muscle ceramide and decrease insulin sensitivity.
Clinical relevance
There are a lot more pathways where ceramide is central to the pathophysiology of the complications including atherosclerosis, myocyte apoptosis and glomerular apoptosis. It seem that ceramides are the unifying culprit in many cardiorenal and metabolic derangements. How does knowing that help us?
DES1
If you refer back to Figure 1, you will see that the enzymes that catalyse the conversion of dihydroceramide to ceramide are DES1 and DES2. This open up a therapeutic window for reducing the production of ceramide just like what the statins do to reduce the production of cholesterol by inhibiting HMG-coA reductase. Indeed, a DES1 inhibitor has been found to be most efficacious in reducing glucose intolerance, fatty liver disease, acute kidney injury and heart failure in mice experiments. Human trials are coming.
CERT score
We also learnt that not all ceramides are the same. Ceramides Cer16:0, Cer18:0 and Cer24:0 are particular potent in causing all the complications we see in these metabolic derangements. These three specific ceramides have been identified as highly linked to cardiovascular disease and insulin resistance. Individuals with elevated plasma ceramides are at higher risk of major adverse cardiovascular events even after adjusting for age, gender, smoking status, and serum biomarkers such as low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol, C-reactive protein (CRP) and lipoprotein-associated phospholipase A2 (Lp-PLA2). And we can now measure these ceramide levels and this lents itself to the development of risk scores that are more robust that our current cardiovascular risk scores. The Mayo clinic in the US is now utilising a CERT score to quantify the cardiovascular risk (4).
In summary, it has been 35 years since statins were first used to reduce cholesterol (especially LDL-C) and have been shown to improve cardiovascular complications. We now have a new kid on the block that seems to be a unifying culprit in these complications. The understanding of the metabolism of ceramides potentially allows us to manipulate ceramide levels to reduce these complications. Further, we will be measuring ceramide levels soon to give us a better risk assessment in patients with cardio-renal and metabolic derangements.
References:
- Galadari S, Rahman A, Pallichankandy S, Galadari A, Thayyullathil F. Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids Health Dis. 2013 Jul 8;12:98. doi: 10.1186/1476-511X-12-98. PMID: 23835113; PMCID: PMC3716967
- Hammerschmidt P, Brüning JC. Contribution of specific ceramides to obesity-associated metabolic diseases. Cell Mol Life Sci. 2022 Jul 5;79(8):395. doi: 10.1007/s00018-022-04401-3. PMID: 35789435; PMCID: PMC9252958.
- Summers SA. Could Ceramides Become the New Cholesterol? Cell Metab. 2018 Feb 6;27(2):276-280. doi: 10.1016/j.cmet.2017.12.003. Epub 2018 Jan 4. PMID: 29307517.
- https://cardiology.testcatalog.org/show/CERAM#:~:text=MI-Heart%20Ceramides%20is%20a%20blood%20test%20that%20measures,adverse%20cardiovascular%20events%20resulting%20from%20unstable%20atherosclerotic%20plaque. Accessed 13th October 2022
