13th October 2022, Dr Chee L Khoo
Patients with type 2 diabetes (T2D) and chronic kidney disease (CKD) are not only more likely to progress towards renal failure requiring dialysis or renal transplant, but also have a greater lifetime risk of cardiovascular (CV) morbidity and mortality (1). This is not surprising though, as both the kidneys and the heart share the same pathophysiology. We have two mineralocorticosteroid receptor antagonists (MRA) available in Australia (spironolactone and eplerenone) although another MRA, finerenone have been available overseas for some time but not in Australia.
Mineralocorticosteroid receptor (MR) is a member of the steroid receptor family of ligand-activated transcription factors. Upon activation by an agonist, the MR initiates the transcription of effector proteins. There are two different MR agonists – aldosterone and cortisol. Activation by aldosterone leads to the expression of protein transporters (mainly the Na+/K+ pump) regulating ionic and water transports and maintains sodium and potassium homeostasis. Activation by cortisol is required for energy homeostasis, stress response and tissue repair following acute injury.
MR is expressed in many tissues, such as the kidney, colon, heart, lung, blood vessels, eye, central nervous system and brown adipose tissue. Overactivation of the MR leads to inflammation and fibrosis in the heart, kidneys and elsewhere where the MR is extensively expressed. This can drive CKD and CV progression and fibrosis in lungs and blood vessels (2).
The MRs are inactivated by MRAs. There are two groups of MRAs – the steroidal MRAs (SMRA) (e.g. spironolactone, eplenerone) and non-steroidal MRAs (e.g. finerenone). The SMRAs have medium to long half-life and have sexual side-effects (e.g. gynaecomastia) although eplerenone has reduced affinity for the androgen and progesterone receptors compared with spironolactone and hence, have less gynaecomastia. Finerenone has no or few sexual side effects, very short half-life and very selective in its binding. It also has no CNS penetration and no active metabolites. The SMRAs are indicated for HFrEF only while finerenone has indication for HFrEF as well as CKD.
The Randomized Aldactone Evaluation Study (RALES) was the first study to demonstrate the efficacy of spironolactone in HFrEF (3). In RALES, patients with severe symptoms (NYHA-III/IV) of heart failure and LVEF ≤35% were randomised to spironolactone or placebo. After a mean follow-up of 24 months, spironolactone was associated with a 30% reduction in all-cause mortality as well as reductions in CV death and heart failure hospitalizations.
In the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) (4) and the Eplerenone in Mild Patients Hospitalizations and Survival Study in Heart Failure (EMPHASIS-HF), eplerenone reduced the composite endpoint of CV death or heart failure hospitalisation by 34% a median follow-up of 21 months in patients with LV dysfunction and NYHA-II symptoms (5).
Finerenone is a novel, selective, nonsteroidal MRA that blocks MR mediated sodium reabsorption and MR overactivation and has demonstrated anti-inflammatory and anti-fibrotic effects in preclinical kidney and CV disease models (6,7).
Two parallel studies conducted to test the efficacy and safety of finerenone published two years ago. The Finerenone in reducing kidney failure and disease progression in Diabetic Kidney Disease (FIDELIO-DKD) (8) and Finerenone in reducing cardiovascular mortality and morbidity in Diabetic Kidney Disease (FIGARO-DKD) (9) phase III trials are complementary in nature due to features such as their similar designs and endpoints. Same drug but one looks at improvement in renal outcomes and the other, improvement in cardiovascular outcomes in similar patients with DKD as their primary outcomes. Together, they form the largest cardiorenal outcomes programme in CKD in type 2 diabetes to date.
Because both trials are essentially looking at similar patients and essentially similar outcomes, it is more useful to looked at the pooled analysis of both of those trials to provide more robust estimates of finerenone efficacy and safety across the spectrum of patients with CKD and type 2 diabetes, to provide reassurance regarding outcomes in a wide range of patients with a degree of precision that was not possible to obtain by considering the two trials separately. This is the basis of the FIDELITY analysis (10).
Patient population
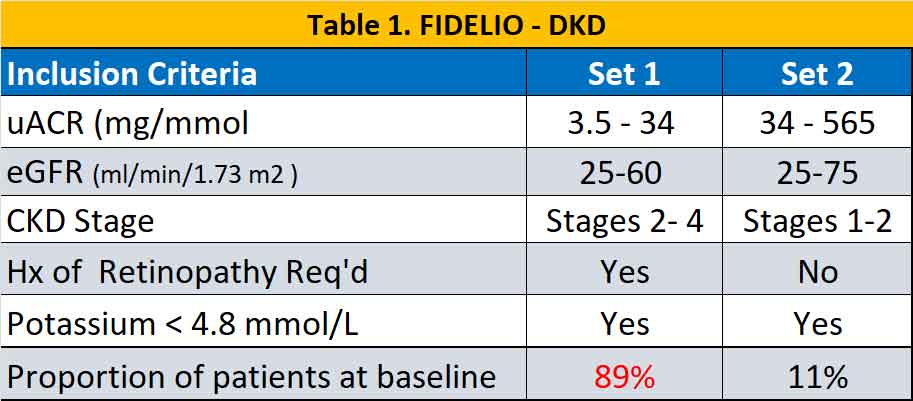
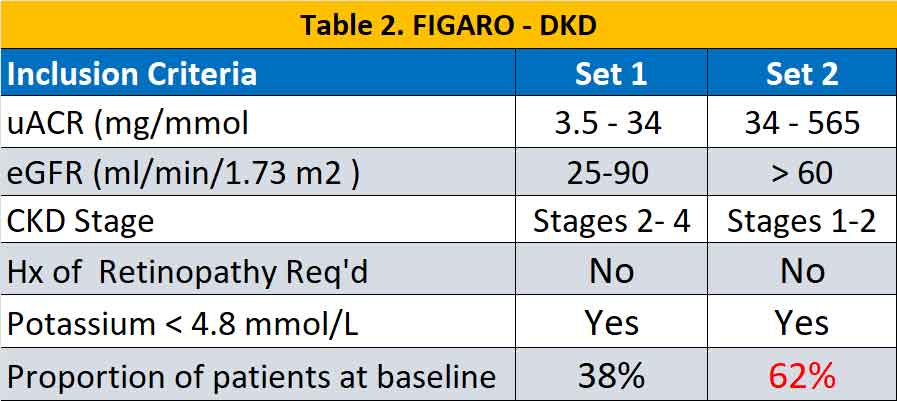
Eligible patients were adults ≥18 years of age with T2D and CKD treated with an ACE inhibitor or ARB at the maximum tolerated dose. A total of 13171 participants were randomised in a 1:1 ratio to receive finerenone or placebo. CKD were defined according to two different sets of criteria and were slightly different in the two studies (see Table 1 & 2). In general, there were more patients with severe CKD (but milder uACR) in the FIDELIO – DKD trial while in FIGARO – DKD there were more patients with milder CKD with higher uACR.
Primary and secondary outcomes
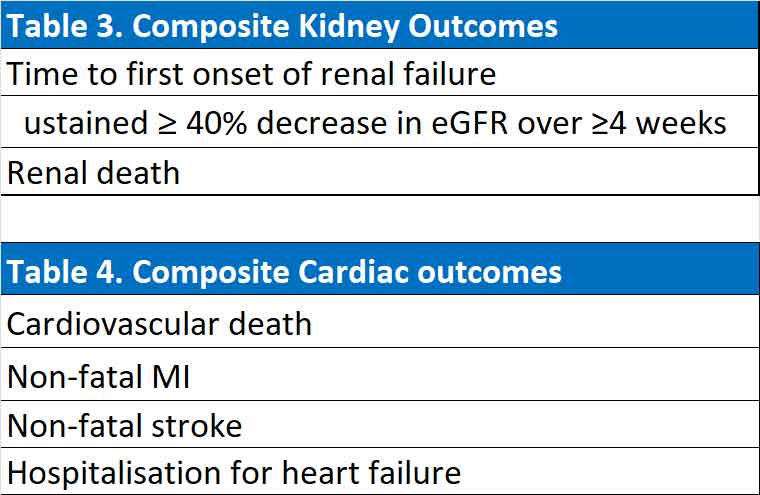
FIDELIO-DKD was primary a renal outcome trial as such the primary outcome was a composite of renal outcomes while the secondary outcome was a composite of cardiovascular outcomes. FIGARO-DKD was primarily a cardiovascular outcome trial and the primary outcome was a composite of cardiovascular outcomes and the secondary outcome was a composite of renal outcomes.
(See Table 3 & 4).
Results
Cardiovascular outcomes
Overall with pooled data from both studies, the mean eGFR was 57.6 ml/min/1.73 m2 with 10.2%, 41% and 48.3% patients with moderate, severe and very severe kidney disease respectively. Finerenone reduced composite cardiovascular outcomes by 14% (p=0.0018). Heart failure hospitalisation were reduced by 22%. Cardiovascular deaths and non-fatal MI were reduced by 14% but non-fatal strokes were similar between the finerenone and placebo groups. The number to treat to prevent a composite cardiovascular event at 3 years was 46. Of note, the cardiovascular benefit from finerenone treatment was similar across all eGFR and uACR levels or whether the patient was on an SGLT2 inhibitor or GLP1 agonist.
Renal outcomes
Finerenone reduced the composite renal outcomes by 23% (p=0.0002). The number to treat to avoid a composite renal outcome at 3 years was 60. If we breakdown the composite renal outcomes, finerenone reduced sustained >57% reduction in eGFR by 30% (p<0.0001), end-stage-kidney-disease by 20% (p=0.040) compared to placebo. All cause mortality and hospitalisation for any cause were similar between the finerenone and placebo groups.
Adverse events
Hyperkalaemia-related adverse events occurred more frequently with finerenone (14.0%) vs placebo (6.9%), but no hyperkalaemia-related adverse events were fatal and only a small proportion led to permanent treatment discontinuation [1.7% (incidence rate 0.66/100 patient-years) and 0.6% (incidence rate 0.22/100 patient-years), respectively] or hospitalisation (0.9% and 0.2%, respectively). Gynaecomastia incidence was low (0.1%) and was similar to placebo (0.2%).
Summary
Based on the FIDELIO-DKD and FIGARO-DKD trial results and the FIDELITY pooled analysis, finerenone is currently indicated in a number of countries to reduce the risk of sustained eGFR decline, ESKD, cardiovascular death, non-fatal MI, and HHF in adult patients with CKD associated with T2D. Across the pooled population, the relative reduction was 14% for the composite cardiovascular outcome and 23% for the composite kidney outcome.
We have spironolactone and eplerenone in Australia for treatment of heart failure with reduced ejection fraction (HFrEF) but their use comes with significant incidence of hyperkalaemia and gynaecomastia which limits our use in patients with HFrEF. Further, eplerenone must be initiated in patients within 14 days of a myocardial infarction.
Finerenone is efficacious in cardiovascular outcomes (like spironolactone and eplerenone) but finerenone trials have demonstrated improvement in kidney outcomes in our patients with diabetes (which has not been shown with spironolactone). As we all know, patients with diabetes have a double whammy of cardiovascular and renal complications and they usually exist in the same patient. You can now see why we anxiously await the arrival of finerenone.
References:
- Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G; FIDELIO-DKD Investigators. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020 Dec 3;383(23):2219-2229. doi: 10.1056/NEJMoa2025845. Epub 2020 Oct 23. PMID: 33264825.
- Agarwal R, Kolkhof P, Bakris G et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J 2021;42:152–161.
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717
- B. Pitt, W. Remme, F. Zannad, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction N Engl J Med, 348 (2003), pp. 1309-1321
- F. Zannad, J.J.V. McMurray, H. Krum, et al. Eplerenone in patients with systolic heart failure and mild symptoms N Engl J Med, 364 (2011), pp. 11-21
- Agarwal R, Kolkhof P, Bakris G et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J 2021;42:152–161.
- Kolkhof P, Jaisser F, Kim SY, Filippatos G, Nowack C, Pitt B. Steroidal and novel non-steroidal mineralocorticoid receptor antagonists in heart failure and cardiorenal diseases: comparison at bench and bedside. Handb Exp Pharmacol 2017; 243:271–305.
- Bakris GL, Agarwal R, Anker SD et al.; FIDELIO-DKD Investigators. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–2229.
- Pitt B, Filippatos G, Agarwal R et al.; FIGARO-DKD Investigators. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021. doi:10.1056/NEJMoa2110956.
- Agarwal R, Filippatos G, Pitt B, et al; FIDELIO-DKD and FIGARO-DKD investigators. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022 Feb 10;43(6):474-484. doi: 10.1093/eurheartj/ehab777. Erratum in: Eur Heart J. 2022 May 21;43(
