12th December 2022, Dr Chee L Khoo
We explored lipoprotein a (Lp(a)) as a significant residual risk factor for atherosclerotic cardiovascular disease (ASCVD) and aortic stenosis in August this year. We looked at the strong and log-linear association between elevated Lp(a) and cardiovascular (CV) events. While the new PCSK9 inhibitors, notably alirocumab, has been shown to modestly reduce Lp(a) levels (and is associated with a small reduction in CV events) in the ODYSSEY trials, we do not have any agents that specifically lower Lp(a) approved yet (1). There are a number of agents in the pipeline, some in Phase 3 trials while others are going through Phase 1 and 2 trials. Reducing Lp(a) levels is one thing but does that reduce CV events? While we don’t have any agents that can substantially reduce Lp(a) levels, there are still a number of risks we can address while we await the new agents’ arrival. Let’s look at what we can do in the meantime.
Lp(a) metabolism
Lipoproteins are produced in the liver but it is unclear how lipoproteins are cleared from the circulation. From animal studies, it appears that the clearance of Lp(a) particles is achieved via the LDL receptor in the liver. In homo- or heterozygous familial hypercholesterolemia patients with a (total) loss of the LDL receptor, Lp(a) plasma levels is elevated, although this may be caused by selection bias (2). Statins do not lower Lp(a) but PCSK9 inhibitors do lower Lp(a), while both upregulate the LDL receptor. This suggests clearance largely independent of the LDL receptor pathway.
Kinetic studies have shown that apo(a) particles are excreted by the kidneys at a steady state. Early in the process of chronic kidney disease, apo(a) excretion is reduced and Lp(a) plasma levels increase (3,4).
Lp(a) levels are primarily genetically determined and are not influenced by lifestyle. Therefore, Lp(a) levels remain stable over life, in contrast to other cholesterol-carrying apoB particles such as low-density lipoprotein (LDL) particles. The Lp(a) plasma concentration is determined by two alleles of the LP(A) gene, coding for the apolipoprotein(a) molecule of the Lp(a) particle.
What is considered elevated Lp(a)?
Depending on the cut-off used, up to 20% of individuals worldwide have elevated Lp(a) plasma levels. Deciding what levels of Lp(a) is consider high is difficult as plasma levels of Lp(a) are highly dependent upon ethnicity [5]. In a recent analysis by Mehta et al. in the MESA (Multi-Ethnic Study of Atherosclerosis), black participants had a median Lp(a) level of 35.2 mg/dl, much higher than white, Hispanic, or Chinese participants (median 13.2 mg/dl) [6]. Despite the racial differences, the ASCVD risk resulting from Lp(a) seems largely similar across different ethnicities [5].
In general, increased risk is suggested to occur > 75 – 125 nmol/L. Your local pathology provider may have a different set of units and range.
Lp(a) and ASCVD and Aortic Stenosis
In one of the Mendelian randomization analyses by the group of Ference, it was shown that every 10 mg/dl (21 nmol/l) Lp(a) increase above median is associated with a 5.8% relative risk increase for coronary artery disease [6]. Elevated Lp(a) is also associated with a high risk of ischaemic stroke and heart failure as well as with cardiovascular and all-cause mortality, albeit with a smaller effect size.
For patients with an Lp(a) above the 95th percentile compared to low levels, the HRs for ischaemic stroke, cardiovascular mortality, and all-cause mortality were 1.6X, 1.5X and 1.2X respectively. In the same population, the HR for calcific aortic valve disease in patients with Lp(a) levels above the 95th percentile is 2.9 X [8].
Mechanisms of damage?
Lp(a) particle contains apoB and may therefore, have similar atherogenic properties as other apoB particles such as LDL-C, although the absolute concentration of Lp(a) particles is usually much lower in comparison with LDL-C. Lp(a) not only is the carrier of LDL-C, it is also the main carrier of oxidised phospholipids (e.g. ceramides). These phospholipids are recognised as damage-associated molecular patterns and therefore can result in pro-inflammatory as well as pro-calcific effects (in aortic valve stenosis). It is postulated that the apolipoprotein(a) part selectively binds to endothelial extracellular matrix proteins and thereby can be retained in the arterial wall [9]. The apolipoprotein(a) part is largely similar in structure to plasminogen which is thrombotic and may interfere with fibrinolysis.
Who should we test?
As to the question of who we should be testing Lp(a) levels on can be a bit tricky. We could order an Lp(a) level for all patients whom we are organising a blood test for. After all, in the ESC 2020 guidelines on management of hyperlipidaemia, they recommend “Lp(a) measurement should be considered at least once in each adult person’s lifetime to identify those with very high inherited Lp(a) levels >180 mg/dL (>430 nmol/L) who may have a lifetime risk of ASCVD equivalent to the risk associated with heterozygous familial hypercholesterolaemia”. This is kind of screening everyone.
But then they also recommend “Lp(a) should be considered in selected patients with a family history of premature CVD, and for reclassification in people who are borderline between moderate and high-risk” (10). This is a little more targeted.
Routine measurement of Lp(a) is not yet recommended in Australia.
Treatment of elevated Lp(a)?
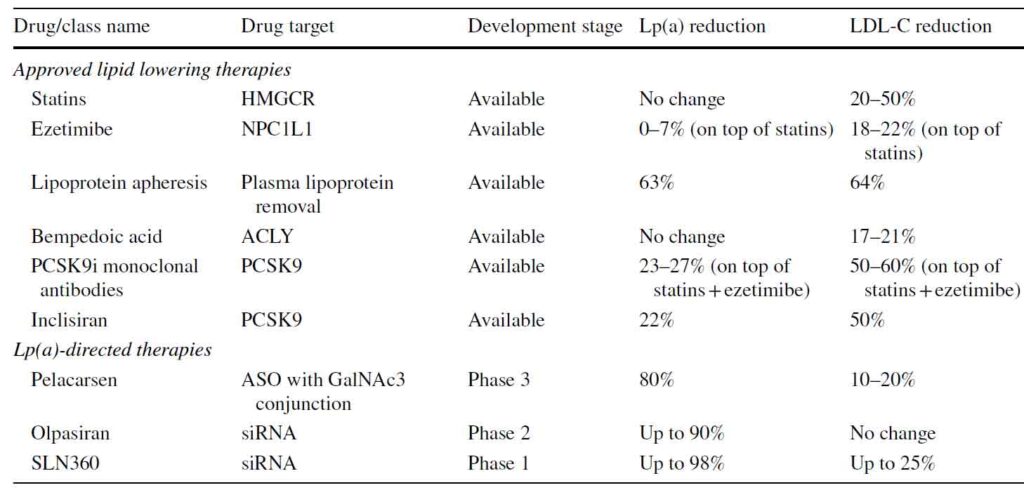
While moderate- to high-intensity statins lower plasma LDL-C for 50% by upregulating the LDL receptor, statins do not lower plasma Lp(a) levels. In fact, a recent meta-analysis of 6 RCTs involving 5256 patients showed that statins significantly increased plasma Lp(a) levels by 11.6% to 24.2% compared to placebo [11). However, a much larger meta-analysis including 24,448 data from 39 placebo controlled RCTs showed no significant effect of statin treatment on Lp(a) plasma levels [12]. Therefore, it does not seem likely that plasma Lp(a) is affected in a clinically meaningful manner by statin therapy. Besides, the benefit from reducing LDL-C overwhelmingly outweigh and potential increase in Lp(a) in patients on statin therapy.
Ezetimibe additionally lowers apoB and LDL-C plasma levels up to 20%, ezetimibe seems to have no or a very small effect on plasma Lp(a) levels. Both as monotherapy as well as in addition to statin therapy, ezetimibe had no effect on plasma Lp(a) concentration in a meta-analysis of 10 placebo-controlled randomised controlled trials (RCTs) including 5188 participants [13].
A third oral lipid lowering drug, bempedoic acid lowers apoB and LDL-C by approximately 20%, depending on the combination of lipid lowering therapies prescribed [14]. Bempdeoic acid was approved by the US FDA and European Medicine Agency in 2020 for the treatment of hypercholesterolemia in combination with diet and the highest tolerated statin therapy in adults with heterozygous familial hypercholesterolemia, or with established atherosclerotic cardiovascular disease, who need additional lowering of LDL cholesterol. There is no data available on the effect of bempedoic acid on Lp(a) levels. Bempedoic acid is not available in Australia.
Both the PCSK9 inhibiting monoclonal antibodies, Alirocumab, Evolocumab and their sister compound, the small interfering RNA (siRNA), Inclisiran have been shown to decrease Lp(a) levels by 25-27%. It is thought that reduction in Lp(a) is likely due to the fact that Lp(a) is metabolised by LDL receptors in the liver. An increase in LDL receptors by PCSK9 inhibitors action will increase the metabolism of Lp(a).
Pelacarsen (TQJ230) is an N-acetylgalactosamine (GalNAc3) conjugated antisense oligonucleotide targeting apolipoprotein(a) mRNA. The phase I/IIa trial proved pelacarsen to be safe and well-tolerated in 64 participants while reducing Lp(a) plasma levels [15]. The subsequent dose-ranging RCT in 286 patients with established ASCVD again showed a mean 80% reduction in plasma Lp(a) levels [16]. The Lp(a)- HORIZON cardiovascular outcome trial have fully enrolled 7680 patients with established ASCVD and due to report in 2025.
Olpasiran is an siRNA agent specifically targeting LP(A) mRNA. In the phase I trial in 64 healthy adults, Lp(a) was persistently reduced up to 90% without major safety issues [17]. Phase 2 is expected to be completed by the end of 2023.
SLN360 is another siRNA agent that has just completed its Phase 1 trial demonstrating that it is well tolerated and reduce Lp(a) by 98%.
Who should we treat?
While we await Lp(a)-HORIZON CVOT to report towards the end of 2025, “treatment” consist of relooking at the patient’s CV risk assessment if Lp(a) is elevated. In patients who may otherwise be deemed as intermediate or higher CV risk, an elevated Lp(a) may warrant initiation of a statin +/- ezetimibe +/- PCSK9i +/- aspirin. If the revised CV risk is now considered high or very high, then the target of LDL-C might have to be revised lower. Perhaps, an LDL-C target of 1.4 mmol/L or lower might now be necessary.
While the US Preventative Services Task Force does not recommend aspirin for primary prevention, a discussion and exploration of the pros and cons of aspirin might be necessary for the patient in front of you. If the bleeding risk is not high or can be mitigated, aspirin for prevention of CV events might be a consideration. This is especially the case, if the patient’s Lp(a) is elevated.
Perhaps, a coronary artery calcium score might come handy to augment the discussion with that patient. We shall explore CAC in the next issue of GPVoice.
References:
- Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018 Nov 29;379(22):2097-2107. doi: 10.1056/NEJMoa1801174. Epub 2018 Nov 7. PMID: 30403574.
- Langsted A, Kamstrup PR, Benn M, Tybjærg-Hansen A, Nordestgaard BG. High lipoprotein(a) as a possible cause of clinical familial hypercholesterolaemia: a prospective cohort study. Lancet Diabetes Endocrinol Elsevier. 2016;4:577–87.
- Speer T, Ridker PM, Von Eckardstein A, Schunk SJ, Fliser D. Lipoproteins in chronic kidney disease: from bench to bedside. Eur Heart J. 2021;42:2170–85.
- Lin J, Reilly MP, Terembula K, Wilson FP. Plasma lipoprotein(a) levels are associated with mild renal impairment in type 2 diabetics independent of albuminuria. PLoS ONE. 2014;9:1–16.
- Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711.
- Mehta A, Vasquez N, Ayers CR, Patel J, Hooda A, Khera A, et al. Independent association of lipoprotein(a) and coronary artery calcification with atherosclerotic cardiovascular risk. J Am Coll Cardiol Am College Cardiol Foundation. 2022;79:757–68.
- Burgess S, Ference BA, Staley JR, Freitag DF, Mason AM, Nielsen SF, et al. 2018 Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a Mendelian randomization analysis. JAMA Cardiol. 2018;3:619–27
- Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. Am College Cardiol Foundation. 2014;63:470–7.
- Boffa MB, Koschinsky ML. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat Rev Cardiol. 2019;16:305–18.
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020 Jan 1;41(1):111-188. doi: 10.1093/eurheartj/ehz455. Erratum in: Eur Heart J. 2020 Nov 21;41(44):4255. PMID: 31504418.
- Tsimikas S, Gordts PLSM, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J. 2020;41:2275–84.
- de Boer LM, Oorthuys AOJ, Wiegman A, Langendam MW, Kroon J, Spijker R, et al. Statin therapy and lipoprotein(a) levels: a systematic review and meta-analysis. Eur J Prev Cardiol. 2021;zwab171.
- Sahebkar A, Simental-Mendía LE, Pirro M, Banach M, Watts GF, Sirtori C, et al. Impact of ezetimibe on plasma lipoprotein(a) concentrations as monotherapy or in combination with statins: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. 2018;8:17887.
- Nurmohamed NS, Navar AM, Kastelein JJP. New and emerging therapies for reduction of LDL-cholesterol and apolipoprotein B: JACC Focus Seminar 1/4. J Am Coll Cardiol Am College Cardiol Foundation. 2021;77:1564–75.
- Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, doubleblind, placebo-controlled, dose-ranging trials. Lancet Elsevier Ltd. 2016;388:2239–53.
- Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif J-C, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med Massachusetts Med Soc. 2020;382:244–55.
- Koren MJ, Moriarty PM, Baum SJ, Neutel J, Hernandez-Illas M, Weintraub HS, et al. Preclinical development and phase 1 trial of a novel siRNA targeting lipoprotein(a). Nat Med. 2022;28:96–103.
