12th September 2023, Dr Chee L Khoo

We have a number of drug classes that are helpful in reducing mortality in patients with heart failure with reduced ejection fraction. But when it comes to heart failure with preserved ejection fraction, SGLT2 inhibitors are the only class that have shown to be of use. ARNI, MRA or betablockers helps with HFrEF but in HFpEF? Nope. What about the GLP1-RAs? The semaglutide people just released a headline suggesting that semaglutide may be “beneficial” in patients with HFpEF. Does it really? Let’s look at the study in detail.
The STEP-HFpEF trial randomised 263 participants to semaglutide 2.4mg weekly and 266 participants to placebo (1). Participants had to be >18 years old, BMI > 30 and had an ejection fraction of >45%. Their 6-min walking distance has to be >100m.
Their Kansas City Cardiomyopathy Questionnaire (KCCQ-CSS) score need to be <90 (the lower the score, the worse the symptoms of HF is) The KCCQ is a standardized, 23-item, participant-administered instrument that quantifies heart failure–related symptoms (frequency, severity, and recent changes), physical function, quality of life, and social function.
HFpEF is confirmed by elevated left ventricular filling pressure, elevated natriuretic peptide plus echocardiographic abnormalities or hospitalisation for heart failure.
Participants were excluded if they had diabetes (HbA1c >6.5%).
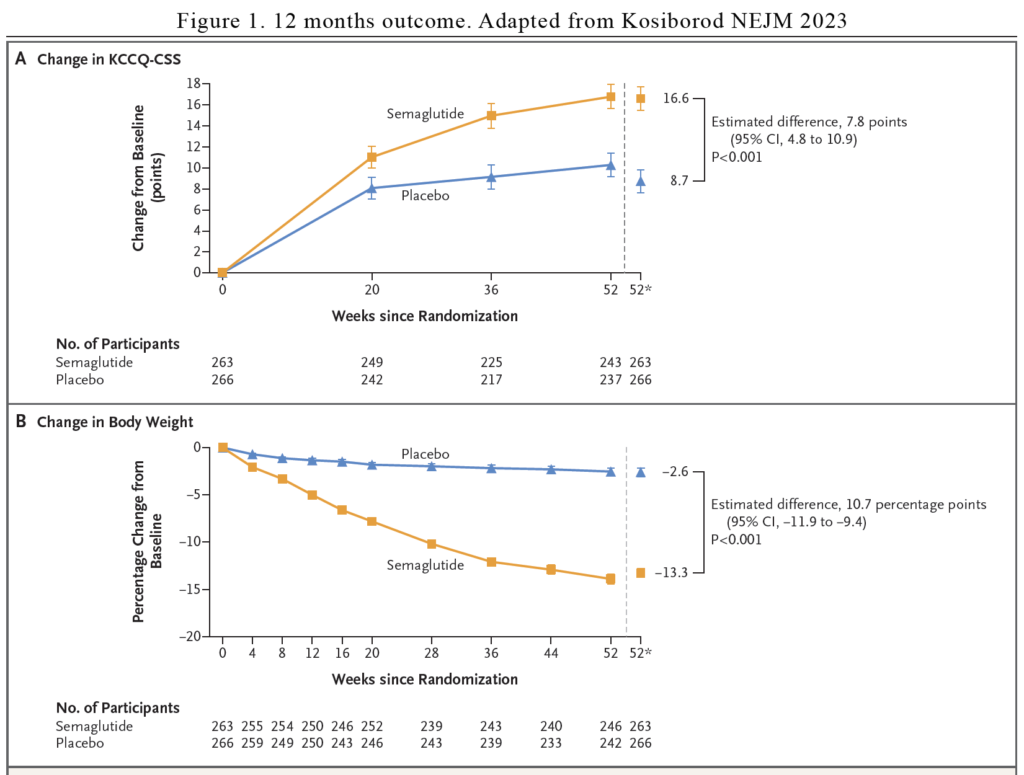
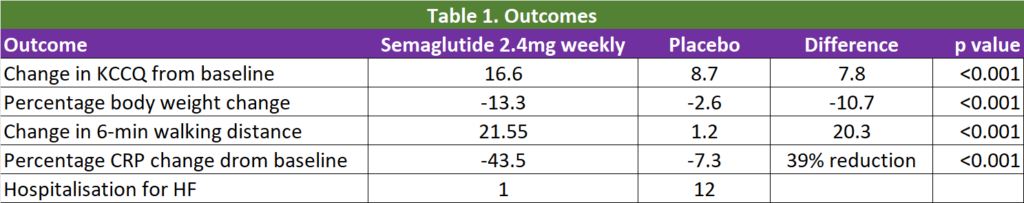
The dual primary end points were the change in the KCCQ-CSS and the percentage change in body weight from baseline to week 52. The secondary end points were change in 6-min walking distance and change in C-reactive protein.
Results – what did they find?
16% of the participants in either group did not complete the treatment but overall 97% of the semaglutide group and 95% of the placebo group completed the trial. 83% of the semaglutide participants received their intended 2.4mg dose while 97% of the placebo group received their intended placebo dose.
The basis of the HF diagnosis were: 14% from elevated filling pressure, 14% from hospitalisation for HF and 72% from elevated NT-proBNP. They all had echocardiographic abnormalities. 96% were white, the median age was 69 years old and the mean BMI was 37. The median KCCQ-CSS were 58.9 points and median 6-min walking distance was 320m.
The median CRP was 3.8 mg/L and 52% of participants had atrial fibrillation, 15% had been hospitalised in the prior 12 months. 66% were in NYHA Class II and 34% in NYHA Class III and IV. Of note only 3.6% were on SGLT2 inhibitors.
After 12 months of study, once weekly 2.4mg semaglutide led to larger reductions in heart failure–related symptoms and physical limitations (as measured with the KCCQ-CSS) and a greater degree of weight loss than placebo at 52 weeks. In addition, semaglutide increased the 6-minute walk distance and reduced CRP to a greater extent than placebo. In total, 1 participant in the semaglutide group and 12 in the placebo group had an adjudicated event of hospitalisation for heart failure or an urgent visit (hazard ratio 0.08). See Figure 1 and Table 1.
What does it all mean?
It is great that there is a study that looks at what is important for patients – relieve of their symptoms (as indicated in the KCCQ) and improvement in their physical state (as indicated by the improvement in the 6-min walking distance). We know that weekly semaglutide lead to significant weight loss compared with placebo (10.7% difference in body weight).
Now, patients with HFpEF are not homogeneous although the majority of them are obese. Adipose tissue may play a pivotal role in the development, progression, and adverse outcomes of heart failure with preserved ejection fraction. The presence of visceral adiposity is associated with increased inflammation, left ventricular hypertrophy, insulin resistance, and diastolic and systolic left ventricular dysfunction, as well as with arterial, skeletal muscle, and physical dysfunction.
Is the improvement with semaglutide the result of the weight loss or the result of semaglutide itself? Certainly, weight loss from semaglutide is a likely contributor to the benefits. Semaglutide may have favourable anti-inflammatory and hemodynamic effects and these may account for some of the benefits. There is a a substantial reduction in the number of participants who were hospitalised for heart failure (HHF) in the semaglutide group compared with placebo but HHF was a secondary outcome and the numbers were low and not powered enough to claim significance. We need clinical trials that are powered to look at that outcome.
Now, it’s easy to run a clinical trial in patients with HFrEF with a primary outcome of improvement in ejection fraction. It’s harder in patients with HFpEF. The hard endpoints in primary outcomes have to be hospitalisation for heart failure or mortality. 12 months is too short a duration to demonstrate those hard endpoints.
In the meantime, in patients with heart failure, an SGLT2 inhibitor is still preferred over a GLP1-RA.
References:
Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, Hovingh GK, Kitzman DW, Lindegaard ML, Møller DV, Shah SJ, Treppendahl MB, Verma S, Abhayaratna W, Ahmed FZ, Chopra V, Ezekowitz J, Fu M, Ito H, Lelonek M, Melenovsky V, Merkely B, Núñez J, Perna E, Schou M, Senni M, Sharma K, Van der Meer P, von Lewinski D, Wolf D, Petrie MC; STEP-HFpEF Trial Committees and Investigators. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N Engl J Med. 2023 Aug 25. doi: 10.1056/NEJMoa2306963. Epub ahead of print. PMID: 37622681.
