10th September 2023, Dr Chee L Khoo

We looked at the new understanding of the pathophysiology of COPD last fortnight. Armed with that information, we may be able to make sense of which puffer to use for which patient and what to escalate during their exacerbations. We already do that but, I must admit, rather haphazardly. It, kind of, depends on what samples I have in the drug cupboard or when the last pharma rep came to sell their ware. We do need a scientific basis to our choice of agents. Where should we get the official information from? The 2023 GOLD guidelines which is 205 pages long (1). Well, we went through a third of the pages last fortnight so not much more to go.
Structural changes
All the insults to the lungs lead to an imbalance between the damage from the inflammatory cells and the epithelial cells trying to counteract that. Protease-mediated destruction of elastin, a major connective tissue component of the lung parenchyma, is an important feature of emphysema but its role may be more difficult to establish in airway changes.
Inflammation may precede the development of fibrosis or repeated injury of the airway wall itself may lead to excessive production of muscle and fibrous tissue. Peribronchiolar fibrosis and interstitial opacities have been reported in patients with COPD and in asymptomatic smokers. The lung vasculature can also be altered in patients with COPD, even those with mild disease.
Chronic inflammation causes structural changes, narrowing of the small airways, luminal exudates in the small airways and destruction of the lung parenchyma that leads to the loss of alveolar attachments to the small airways and decreases lung elastic recoil. A loss of small airways may also contribute to airflow obstruction and mucociliary dysfunction.
Thus, airflow obstruction is caused by a mixture of small airways disease (which increases airway resistance) and parenchymal destruction (emphysema, that reduces the normal elastic recoil of the lung parenchyma), the relative contributions of which vary from person to person. Further, these changes do not always occur together and may evolve at different rates over time.
All these changes limit emptying of the lungs during forced expiration, decrease FEV1 and the FEV1/FVC ratio, and contribute to gas trapping and lung hyperinflation.
Structural abnormalities in the airways, alveoli and pulmonary circulation in patients with COPD alter the normal ventilation-perfusion (VA/Q) distributions. This is the main mechanism of abnormal pulmonary gas exchange resulting in different degrees of arterial hypoxemia, without or with hypercapnia.
As you can see, in COPD, there is airway narrowing, airway inflammation and potentially, hypoxia. The goal of treatment is:
- Reduce symptoms to improve patient’s quality of life
- Reduce exacerbation as exacerbation hasten lung function decline
- Reduce mortality – pulmonary and non-pulmonary
Bronchodilators – which one or ones?
Bronchodilators act by altering airway smooth muscle tone and the improvements in expiratory flow reflect widening of the airways. They tend to reduce dynamic hyperinflation at rest and during exercise and improve exercise performance. Increasing the dose of either a beta2-agonist or an anticholinergic appears to provide subjective benefit in acute episodes but is not necessarily helpful in stable disease.
The long-acting beta agonists (LABA) work by stimulating beta2-adrenergic receptors. Some are given once a day (Indacaterol, Oladaterol and Vilanterol) while others are given twice a day (Formoterol and Salmeterol). In general, they improve lung function, improve symptoms and reduce exacerbations.
The long acting anti-muscarinic drugs (LAMA) work by blocking acetylcholine on M3 muscarinic receptors expressed in airway smooth muscle. Tiotropium and umeclidinium are given once a day while aclidinium is given twice a day. Glycopyrronium can be given once or twice a day. Like the LABA, LAMA improve lung function and symptoms and reduce exacerbations.
Clinical trials show that LAMA are better than LABA in reducing exacerbation (2,3). Use of short acting bronchodilators on a regular basis is not generally recommended.
Combination LAMA/LABA bronchodilators
Combining bronchodilators with different mechanisms and durations of action may increase the degree of bronchodilation with a lower risk of side-effects compared to increasing the dose of a single bronchodilator. These combinations improve lung function compared to placebo(110); this improvement is consistently greater than long acting bronchodilator monotherapy effects although the magnitude of improvement is less than the fully additive effect predicted by the individual component responses. Most studies with LABA+LAMA combinations have been performed in patients with a low rate of exacerbations.
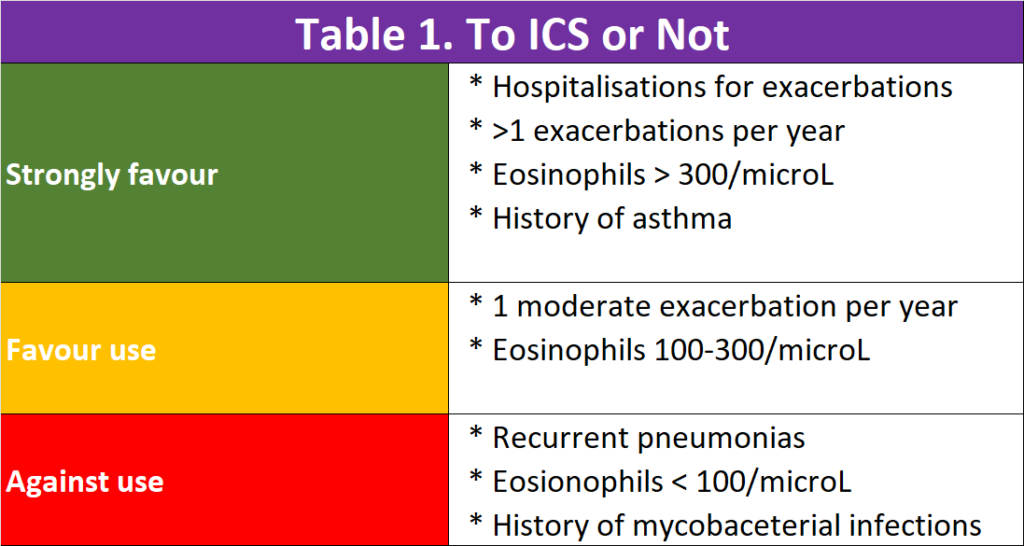
To ICS or not to ICS
We need inhaled corticosteroid (ICS) to reduce the airway inflammation. While ICS is commonly used alone in patients with asthma, regular treatment with ICS alone does not modify the long-term decline of FEV1 nor mortality in people with COPD.
Triple inhaled therapy of LABA+LAMA+ICS improves lung function, symptoms and health status, and reduces exacerbations, compared to LABA+ICS, LABA+LAMA or LAMA monotherapy (Evidence A). Recent data suggest a beneficial effect of triple inhaled therapy versus fixed-dose LABA+LAMA combinations on mortality in symptomatic COPD patients with a history of frequent and/or severe exacerbations.
There were suggestions that regular treatment with ICS increases the risk of pneumonia especially in those with severe disease. This is a worry.
In patients with COPD, lower blood and sputum eosinophils are associated with greater presence of proteobacteria, notably Haemophilus, increased bacterial infections & pneumonia. These patients have a higher risk of pneumonia.
There is a continuous relationship between blood eosinophil counts and ICS effects; no and/or small effects are observed at lower eosinophil counts, with incrementally increasing effects observed at higher eosinophil counts. Data modelling indicates that ICS containing regimens have little or no effect at a blood eosinophil count < 100 cells/μL, therefore this threshold can be used to identify patients with a low likelihood of treatment benefit with ICS. The treatment effect of ICS containing regimens (LABA+LAMA+ICS and LABA+ICS vs LABA+LAMA) is higher in patients with high exacerbation risk (= 2 exacerbations and / or 1 hospitalization in the previous year (4-6).
So, triple therapy inhaler is not always better than double therapy inhalers. See Table 1.
Reduce mortality?
Yes, the bronchodilators +/- ICS improves lung function and symptoms and reduce exacerbations. Remember, with each exacerbation, there is further decline in lung function. Does it reduce mortality? It should, shouldn’t it? The data have been rather disappointing up until recently. The IMPACT and ETHOS trials suggested a 28% and 49% reduction in all-cause mortality respectively, compared with dual therapy in patients with severe COPD (7,8). Not everyone agrees on the robustness of the results. Patients on double therapy had their ICS withdrawn at the beginning of the trials. Perhaps, ICS withdrawl contributed to worsening results in the double therapy group. Mortality rates were low in both groups and was only a secondary outcome and the numbers may not be powered for significance.
In summary, lung function in patients with COPD, like all chronic illness, progressively decline. Bronchodilators not only improve symptoms but they also reduce exacerbations and may reduce mortality. Single inhaler is better than multiple inhalers. LAMA + LABA is better than monotherapy. LAMA+LABA+ICS may be suitable for patients with severe COPD who are not at high risk of pneumonia. Blood eosinophils may help us choose who might benefit.
References:
- https://goldcopd.org/2023-gold-report-2/. Accessed on 22nd August 2023.
- Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax 2001; 56(11): 880-7.
- Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987; 42(10): 773-8.
- Boutou AK, Shrikrishna D, Tanner RJ, et al. Lung function indices for predicting mortality in COPD. Eur Respir J 2013; 42(3): 616-25.
- de-Torres JP, O’Donnell DE, Marin JM, et al. Clinical and Prognostic Impact of Low Diffusing Capacity for Carbon Monoxide Values in Patients With Global Initiative for Obstructive Lung Disease I COPD. Chest 2021; 160(3): 872-8.
- Puente-Maestu L, Palange P, Casaburi R, et al. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J 2016; 47(2): 429-60
- Lipson DA, Barnhart F, Brealey N, et al; IMPACT Investigators. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671-1680. doi: 10.1056/NEJMoa1713901.
- Martinez FJ, Rabe KF, Ferguson GT, Wedzicha JA, Singh D, Wang C, Rossman K, St Rose E, Trivedi R, Ballal S, Darken P, Aurivillius M, Reisner C, Dorinsky P. Reduced All-Cause Mortality in the ETHOS Trial of Budesonide/Glycopyrrolate/Formoterol for Chronic Obstructive Pulmonary Disease. A Randomized, Double-Blind, Multicenter, Parallel-Group Study. Am J Respir Crit Care Med. 2021 Mar 1;203(5):553-564. doi: 10.1164/rccm.202006-2618OC. PMID: 33252985; PMCID: PMC7924571.
