27th May 2025, A/Prof Chee L Khoo
Hypertriglyceridaemia (HTG) causes and contribute to a number of serious medical conditions including pancreatitis, cardiovascular disease, MAFLD and worsening of type 2 diabetes. The efficacy and treatment options are complicated. Apart from treating any secondary causes, our treatment options are quite limited. Marine-derive omega-3 fish oils, fenofibrate and the new icosapent ethyl are all we have in our tool kit. Angiopoietin-related protein 4 (ANGPTL4) inhibition is looking very promising after some false starts. MAR001 has just completed Phase1/2a safely. Let’s look at this new class of medication.
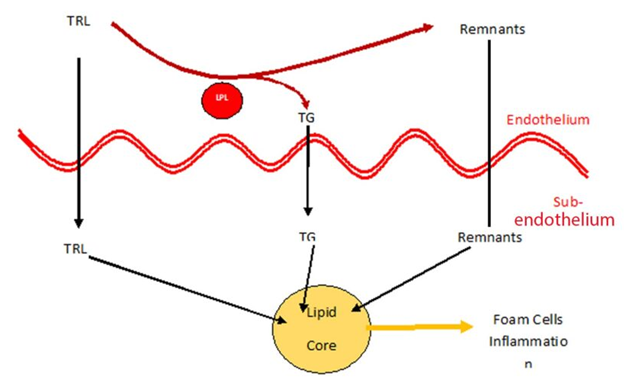
We looked at the mechanisms of damage and the relationship between triglycerides and the various lipoproteins in ASCVD extensively last year when icosapent ethyl first came on the PBS. We know that HTG is an independent risk factor in ASCVD. Essentially, triglycerides (TG) are cleaved off the TG rich lipoprotein (TRL) by endothelial surface located lipoprotein lipase leaving behind TG rich remnants. Normally, the TG cleaved from the TRL is then removed from plasma and stored away. See Figure 1.
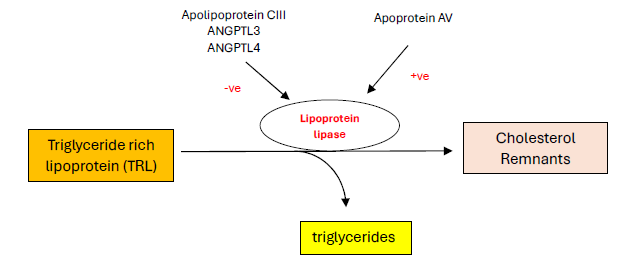
The action of LPL is regulated by negatively by inhibitors (Apolipoprotein CIII, ANGPTL3, ANGPTL4) and positively by Apoprotein AV. In other words, ANGPTL4 is an inhibitor of lipoprotein lipase and inhibiting ANGPTL4 will enhance the activity of LPL and increase the lipolysis of TRL and theoretically, reduce plasma TG. See Figure 2.
Data from human genetic studies strongly support ANGPTL4 as a key regulator of lipid metabolism and a promising therapeutic target for ASCVD risk reduction. Human carriers of loss-of-function alleles in ANGPTL4 show decreased plasma triglycerides, remnant cholesterol, and apolipoprotein B concentrations, favourable body composition with augmented gluteo-femoral fat mass, and reduced risk of both type 2 diabetes and ASCVD (1-4).
However, when ANGPTL4-knockout mice were fed a high saturated fat diet, there were lipid accumulation in mesenteric lymph nodes, mesenteric lymphadenitis, rapid-onset and persistent local and systemic inflammation, and decreased survival (5,6).
Fortunately, individuals with ANGPTL4 loss-of-function showed no evidence of mesenteric lymphadenitis, systemic inflammation, or related adverse effects, even when consuming co-called Western diets and having a BMI greater than 30 kg/m². Abdominal MRIs from homozygous loss-of-function carriers showed absence of lymphatic abnormalities and electronic medical record analysis from homozygous carriers of the ANGPTL4 (2,4,7).
As a result, the development of ANGPTL4 inhibitors have been cautious and delayed. We now have the first in-human randomised placebo-controlled Phase1 trial reporting (8).
MAR001 (developed by Marea Therapeutics, San Francisco) is a monoclonal antibody which inhibit ANGPTL4. In a first-in-human, randomised, placebo-controlled, single-ascending-dose phase 1 study healthy participants received a single subcutaneous injection of MAR001 or placebo:
- In part 1A, participants were healthy men and women aged 18–65 years with a bodyweight of at least 50 kg and a BMI of 18–30 kg/m².
- In part 1B, participants weighed at least 70 kg and had a BMI of 30–40 kg/m².
- In part 1C, participants weighed at least 59 kg and had a fasting triglyceride concentration of 1.7-5.6 mmol/L at the screening visit.
Because these are Phase 1 (safety) trials, the primary objectives of the first-in-human single-dose clinical study were to assess the safety and tolerability of a single subcutaneous injection of MAR001. The secondary outcomes then become part of an efficacy trial as they looked at postprandial triglycerides in response to a meal tolerance test and fasting lipids, including triglyceride, total cholesterol, LDL cholesterol, and HDL cholesterol.
MAR001 was also assessed in a randomised, double-blind, placebo-controlled phase 1b/2a study in participants with metabolic dysfunction. The study was done at two sites in Australia.
Participants were adults with:
- TG 1.7 – 5.6 mmol/L
- T2D or evidence of insulin resistance and
- Obese (based on waist circumference criteria).
The primary objective was the safety and tolerability of multiple doses of MAR001. They also looked at similar secondary objectives.
Results
In the first trial Phase 1 trial, 12 weeks of MAR001 treatment did not result in changes to MRI measurements of mesenteric lymph nodes, including lymph node number, size, or new mesenteric inflammation or ascites. There was also no apparent dose-related trend in gastrointestinal adverse events, and no increases in systemic inflammation.
In the randomised controlled studies, MAR001 treatment led to substantial reductions in triglycerides and remnant cholesterol. MAR001 resulted in a placebo-adjusted reduction of 68·6% in triglycerides and 65·6% in remnant cholesterol. In the multidose study, baseline triglyceride concentrations were lower (220 mg/dL in the 450 mg group) and MAR001 reduced triglyceride by 52·7% and remnant cholesterol by 52·5%. The TG reductions were accompanied by reductions in non-HDL cholesterol, with an increase in HDL cholesterol, whereas total cholesterol and LDL cholesterol concentrations remained unchanged.
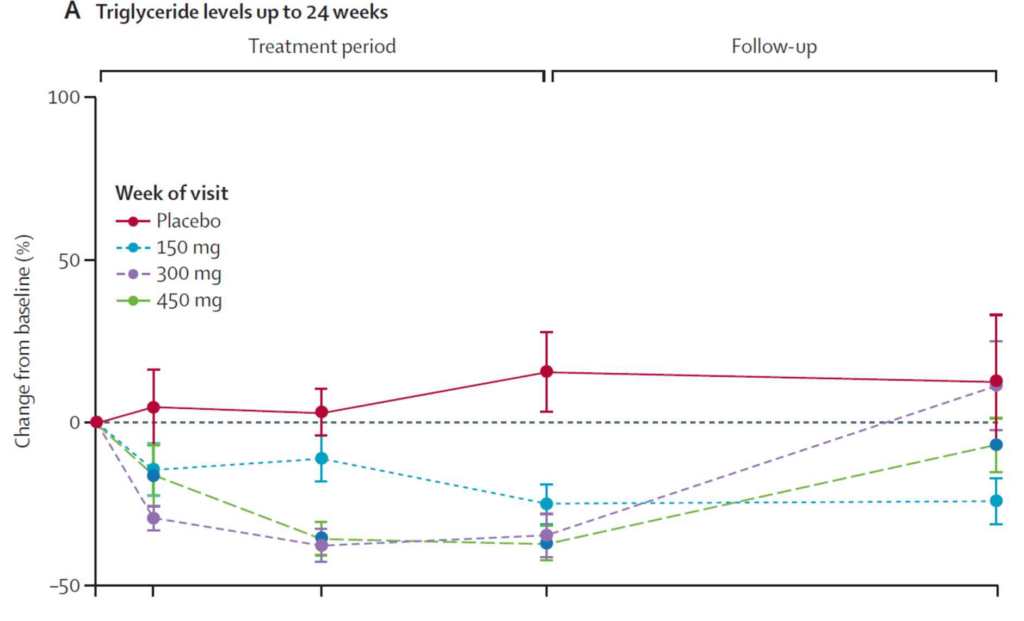
While fish oil supplementation may or may not lead to reductions in TG levels, MAR001 appears to at least 50% reduction in TG. Whether this reduction in TG will translate to improvement in CV outcomes remains to be seen. We need to be mindful that large-scale clinical trials of triglyceride-rich lipoprotein-lowering therapies have not convincingly shown an improvement in outcomes (32).
References:
- Richardson TG, Leyden GM, Wang Q, et al. Characterising metabolomic signatures of lipid-modifying therapies through drug target mendelian randomisation. PLoS Biol 2022; 20: e3001547.
- Landfors F, Henneman P, Chorell E, Nilsson SK, Kersten S. Drug-target Mendelian randomization analysis supports lowering plasma ANGPTL3, ANGPTL4, and APOC3 levels as strategies for reducing cardiovascular disease risk. Eur Heart J Open 2024; 4: oeae035.
- Wang Q, Oliver-Williams C, Raitakari OT, et al. Metabolic profiling of angiopoietin-like protein 3 and 4 inhibition: a drug-target Mendelian randomization analysis. Eur Heart J 2021; 42: 1160–69.
- Gagnon E, Bourgault J, Gobeil É, Thériault S, Arsenault BJ. Impact of loss-of-function in angiopoietin-like 4 on the human phenome. Atherosclerosis 2024; 393: 117558.
- 19 Desai U, Lee E-C, Chung K, et al. Lipid-lowering effects of antiangiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci USA 2007; 104: 11 766–71.
- Lichtenstein L, Mattijssen F, de Wit NJ, et al. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metab 2010; 12: 580–92.
- Dewey FE, Gusarova V, O’Dushlaine C, et al. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med 2016; 374: 1123–33.
- Cummings BB, Joing MP, Bouchard PR, et al. Safety and efficacy of a novel ANGPTL4 inhibitory antibody for lipid lowering: results from phase 1 and phase 1b/2a clinical studies. Lancet. 2025 May 15:S0140-6736(25)00825-6.
