27th January 2026, A/Prof Chee L Khoo

We are very familiar with preventing the progression of prediabetes to type 2 diabetes (T2D). Achievement of remission involves multi-component interventions including dietary and physical activity measures and often, pharmacological intervention. Patients with T2D are at heightened cardiometabolic complications. You would think that preventing the progression from prediabetes to diabetes will prevent some of these complications. Now, prediabetes is already associated with atherosclerotic cardiovascular disease, heart failure, and premature death (1,2) before they progressed to full blown T2D. Will diabetes prevention reduce these complications? Surprisingly, the evidence is rather conflicting. Maybe we need these patients with prediabetes to have normalised glucose profile to achieve benefits. We need prediabetes remission.
We need to clarify the diagnostic criteria for prediabetes. The ADS, WHO and IDF differ from the ADA in what is prediabetes (12,13). See Table 1. The distinction is important as they are a number of different diabetes prevention programs across different countries and the patient population will be different.
There have been quite a few diabetes prevention programs internationally over the last 30 years. All have demonstrated that lifestyle intervention can significantly decrease the progression from prediabetes to T2D. The studies continue to monitor these patients over that period of time and we now have cardiovascular and mortality data available. The DaQing Diabetes Prevention Outcomes study (DaQingDPOS) began in 1986 in Daqing, China, and found that 6 years of structured lifestyle intervention (diet, exercise, or both) in individuals with prediabetes was associated with reductions in cardiovascular mortality by 33% and all-cause mortality by 26% over three decades of followup, with the magnitude of benefit closely related to the duration of time spent free from type 2 diabetes (3-5).
By contrast , the US-based, the Diabetes Prevention Program Outcomes study (DPPOS), the long-term follow-up of the Diabetes Prevention Program (DPP) did not observe statistically significant reductions in major adverse cardiovascular events (MACE) or mortality after 21 years (6). The Finnish Diabetes Prevention Study came to similar negative conclusion (7). When we combined these RCTs in a meta-analysis, the overall conclusion was lifestyle intervention in these trials did not translate to improvement in CV or overall mortality (8). Obviously, there will be a lot of confounding factors which differ in these studies e.g. how aggressive the lipids and BP were managed, how much weight loss were achieved and the duration of diabetes.
Perhaps, preventing progression to T2D may not be enough. We may need to achieve normalised glycaemic levels. That is fasting glucose of < 5.6 mmol, 2-hr post prandial glucose < 7.8 mmol/L or HbA1c of < 5.7%. A major component of the lifestyle measures is weight loss. When one implement lifestyle measures, with or without weight loss, we also achieve improved insulin sensitivity, beta-cell function, reduced visceral adiposity and greater protection against type 2 diabetes onset compared with non-remission (9-10). We have evidence from PLIS demonstrating that remission from prediabetes to normal glucose regulation improves both insulin sensitivity and visceral fat distribution despite similar weight loss (9). We know that heart failure and cardiovascular disease are closely linked to insulin resistance and excess visceral adiposity. While preventing progression to T2D may not always translate to improvement in CV and overall mortality, restoring normoglycaemia may.
Vazquez Arreola, E et al performed a post-hoc analyses from two landmark diabetes prevention trials, the US Diabetes Prevention Program Outcomes Study (DPPOS) and the Chinese DaQing Diabetes Prevention Outcomes Study (DaQingDPOS) after 20 and 30 years respectively (11). They investigated whether prediabetes remission was associated with a lower incidence of cardiovascular death or hospitalisation for heart failure compared with non-remission, with a long-term legacy effect.
The DPP trial compared the effects of an intensive lifestyle intervention with the aim of reducing weight by 7% and increasing exercise time to 150 min per week, metformin, or placebo on the prevention or delay of type 2 diabetes in participants with a BMI of 24 kg/m² or greater (≥22 kg/m² if Asian) (5).
For the DaQingDPOS, 576 people aged 25–74 years with impaired glucose tolerance were recruited. People with previous cardiovascular or cerebrovascular disease were excluded. Participants were randomly assigned to one of three interventions—diet, exercise, or diet plus exercise—or to the control group receiving no specific lifestyle programme. Intensive lifestyle interventions were conducted for 6 years until 1992, with regular counselling and follow-up of more than 30 years.
This post hoc analysis compared the primary outcome of cardiovascular death or hospitalisation for heart failure over 20 (DPPOS) and 30 years (DaQingDPOS) between those who achieve prediabetes remission versus those that didn’t.
The DPPOS results
individuals reaching remission were younger and had lower glucose levels at baseline than individuals not in remission, median 5·7 mmol/l (IQR 5·5–5·9) versus 5·8 mmol/l (5·6–6·2), p<0·0001). People reaching remission had lower type 2 diabetes incidence (2·36 cases per 100 person years) than those not reaching remission (5·41 cases per 100 person years p <0·0001). Follow-up for type 2 diabetes development was higher in people who reached remission (median 15·0 years [IQR 8·0–21·5]), compared with people who had not (median 8·2 years [3·5–19·0];
The median follow up times were similar for both groups (~20 years). The crude and the fully adjusted hazard ratios (HR) comparing people reaching prediabetes remission to those not reaching remission were 0·41 (95% CI 0·20–0·84; p=0·014) versus 0·47 (95% CI 0·23–0·97; p=0·041. The crude HR for extended MACE comparing people reaching prediabetes remission to those not reaching remission was 0·63. The crude HR for mortality comparing people who reached prediabetes remission to those not reaching remission was 0·65 (95% CI 0·44–0·96; p=0·030) and the adjusted HR was 0·78 (0·53–1·16; p=0·226). The analysis shows that remission at least once during followup was significantly associated with reduced risk for most cardiovascular outcomes. the HR for the composite event of cardiovascular death or hospitalisation for heart failure comparing participants who reached remission according to ADA criteria at least once during followup with participants who did not was 0·43.
The DaQingDPOS results
Of the 576 participants with prediabetes, 36 were lost to follow-up. The median follow up was > 30 years. The crude HR (remission compared with non-remission) was 0·53 (95% CI 0·31–0·90; p=0·019) for the composite endpoint of cardiovascular death or hospitalised heart failure, 0·58 (0·33–1·03; p=0·062) for cardiovascular death, 0·44 (0·19–1·00; p=0·050) for hospitalisation for heart failure, 0·56 (0·40–0·78; p=0·004) for MACE, and 0·59 (95% CI 0·40–0·89; p=0·011) for mortality. Even after adjustment for age, sex, BMI, systolic blood pressure, smoking status, cholesterol, intervention, use of medications (insulin or oral glucose lowering agents, blood pressure lowering, and lipid lowering medication), change of body weight at the end of year 6, and the time dependent diabetes after completion of the 6year intervention, the HRs were of similar magnitude and significance.
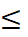
When you combined both datasets in a pooled analysis, the pooled adjusted HR of cardiovascular death or hospitalisation for heart failure was 0·63 (0·47–0·84, p=0·0014; I²=62%), when comparing people reaching remission with people not reaching remission by ADA criteria. Because not all the three parameters of glucose is available in primary care, they use fasting glucose as a proxy for remission to normal glycaemia. The lowest FPG value associated with a statistically significant risk reduction for both endpoints was 5.3mmol/L, compared with participants who did not reach this threshold.
When the original DPPOS first reported, there was no overall improvement in cardiovascular or mortality benefit. One of the explanations were perhaps, the extensive use of lipid and BP lowering medications and metformin may have diluted the results. This study has highlighted a number of important conclusions:
- We may have need to go all the way to achieve normoglycaemia to achieve CV and mortality benefits
- Early attainment of remission (after 1 year in the DPPOS and 6 years in the DaQIngDPPOS) have a legacy effect on cardiovascular outcomes 2-3 decades later.
- Being in remission once during the decade long follow up was linked to reduction in cardiovascular outcomes.
- It’s not just weight reduction to prevent progression to T2D we need to aim for. These patients need a multi-component lifestyle intervention to achieve cardiovascular benefits.
Let’s not just prevent progression of prediabetes to T2D. Let’s aim for normoglycaemia. Let’s aim for prediabetes remission.
References:
- Rooney MR, Wallace AS, Echouffo Tcheugui JB, et al. Prediabetes is associated with elevated risk of clinical outcomes even without progression to diabetes. Diabetologia 2025; 68: 357–66. Sandforth L, Kullmann S, Sandforth A, et al. Prediabetes remission to reduce the global burden of type 2 diabetes. Trends Endocrinol Metab 2025; 36: 899916.
- American Diabetes Association Professional Practice Committee; 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 1 January 2024; 47 (Supplement_1): S20–S42.
- Li G, Zhang P, Wang J, et al. Cardiovascular mortality, allcause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23year followup study. Lancet Diabetes Endocrinol 2014; 2: 474–80.
- Gong Q, Gregg EW, Wang J, et al. Longterm effects of a randomised trial of a 6year lifestyle intervention in impaired glucose tolerance on diabetesrelated microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia 2011; 54: 300–07.
- Gong Q, Zhang P, Wang J, et al, and the Da Qing Diabetes Prevention Study Group. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol 2019; 7: 452–61.
- Goldberg RB, Orchard TJ, Crandall JP, et al, and the Diabetes Prevention Program Research Group. Effects of longterm metformin and lifestyle interventions on cardiovascular events in the Diabetes Prevention Program and its outcome study. Circulation 2022; 145: 1632–41.
- Uusitupa M, Peltonen M, Lindström J, et al, and the Finnish Diabetes Prevention Study Group. Tenyear mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study—secondary analysis of the randomized trial. PLoS One 2009; 4: e5656.
- Rutters F, den Braver NR, Lakerveld J, et al. Lifestyle interventions for cardiometabolic health. Nat Med 2024; 30: 3455–67
- Sandforth A, von Schwartzenberg RJ, Arreola EV, et al. Mechanisms of weight lossinduced remission in people with prediabetes: a posthoc analysis of the randomised, controlled, multicentre Prediabetes Lifestyle Intervention Study (PLIS). Lancet Diabetes Endocrinol 2023; 11: 798–810.
- Sandforth A, Arreola EV, Hanson RL, et al. Prevention of type 2 diabetes through prediabetes remission without weight loss. Nat Med 2025; 31: 3330–40.
- Vazquez Arreola E, Gong Q, Hanson RL, Wang J, Sandforth L, He S, Sandforth A, Qian X, Giacca M, Bornstein SR, Fritsche A, Stefan N, Preissl H, Gregg EW, Marx N, Jumpertz-von Schwartzenberg R, Li G, Birkenfeld AL. Prediabetes remission and cardiovascular morbidity and mortality: post-hoc analyses from the Diabetes Prevention Program Outcome study and the DaQing Diabetes Prevention Outcome study. Lancet Diabetes Endocrinol. 2026 Feb;14(2):137-148. doi: 10.1016/S2213-8587(25)00295-5. Epub 2025 Dec 12. PMID: 41397402.
- https://www.diabetessociety.com.au/downloads/20200714%20A%20Position%20Statement%20on%20Screening%20and%20Management%20of%20Prediabetes%20in%20Adults%20in%20Primary%20Care%20in%20Australia%20-%20updated.pdf. Accessed 25/1/2026
- https://diabetesatlas.org/media/uploads/sites/3/2025/04/IDF_Atlas_11th_Edition_2025-1.pdf. Accessed 25/1/2026
