8th December 2019, Dr Chee L Khoo
Anaemia is quite common in patients with chronic kidney disease (CKD). Disruption in the production of erythropoietin is not the only cause. Iron deficiency, uremia, chronic inflammation and gastrointestinal (G) loss are the common the other causes of anaemia in these patients. After excluding GI losses, current treatment consists of iron replacement and the use of erythropoietin stimulating agents (ESA).
How common is anaemia in CKD?
CKD anaemia is diagnosed when Hb is less than 13.0 g/dL in adult males and less than 12.0 g/dL in adult females. While anaemia can be present in patients in all stages of CKD, the prevalence increases as the CKD progresses. It is present in 8.4% of patients with CKD 1 and up to 53.4% in patients with CKD 5. See Figure 1. (7). In patients without anaemia, it is recommended that patients with CKD 3 be screened for anaemia at least annually while patients with CKD 4-5 at least twice a year. In patients with anaemia and CKD 3-5, screening at least 3-monthly is appropriate.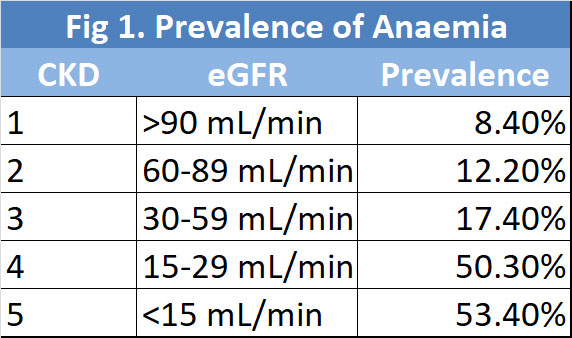
What are the consequences of anaemia in CKD?
Anaemia in patients with CKD is not just about fatigue and tiredness. CKD anaemia is associated with increased cardiovascular hospitalisation and mortality,. The more severe the CKD is, the higher those risks are (8).
Who needs treatment?
Erythropoietin stimulating agents (ESA) should be considered only when Hb drops to <10.0 g/dL. The target Hb should be 11.5 g/dL as higher targets have been linked to increased cardiovascular events. The FDA has issued warnings on the use of ESA due to a greater risk of death and serious cardiovascular events. It makes sense though, when you think about it. If you increase Hb with ESA, you may increase the viscosity of blood and CV events may increase. How quickly and how much you increase the Hb then affects the safety of the therapy.
Hypoxia-inducible-factor (HIF)
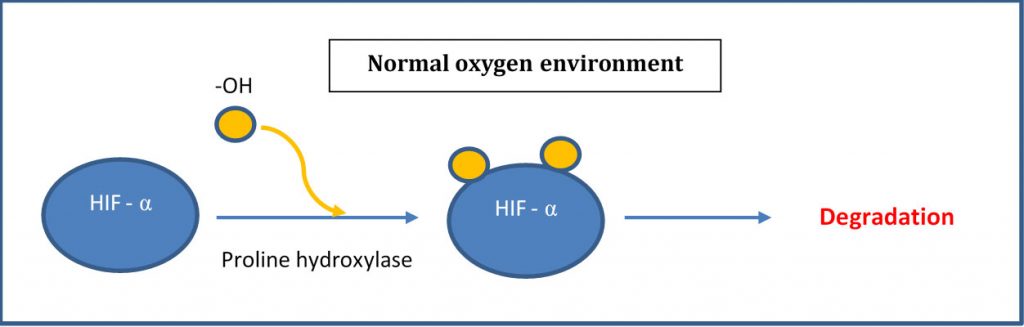
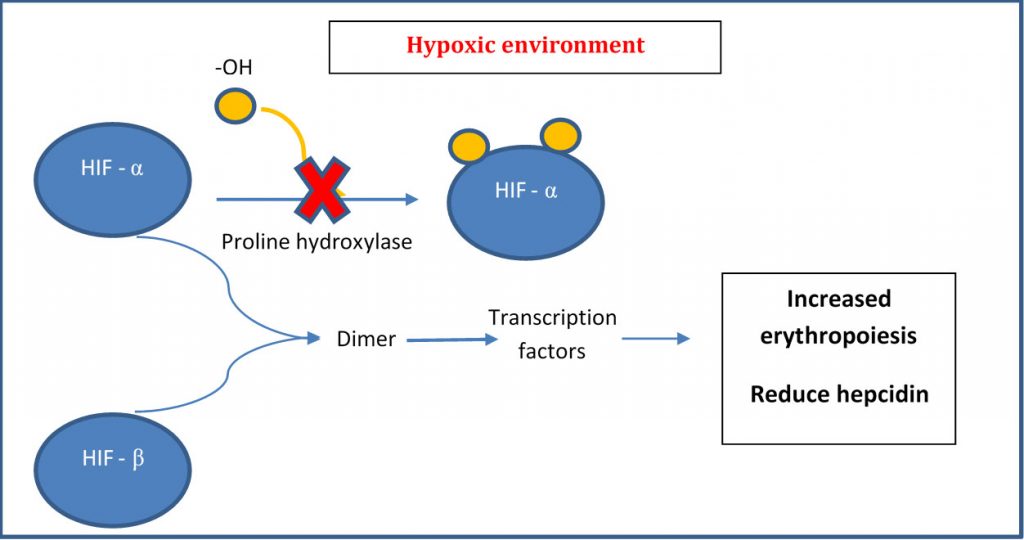
Under normoxic (normal oxygen) conditions, HIF-α is hydroxylated by a prolyl hydroxylase and subsequently degraded. Under hypoxic (low oxygen) conditions, no oxygen is available to the hydroxylation and the excess HIF- α moves intracellularly and translocate into the nucleus to form a dimer with HIF-β. This dimer complex then binds to the HRE (hypoxia responsive element) within the nucleus resulting in the transcription of genes involved in a multitude of processes including erythropoiesis, glycolysis and angiogenesis (see below) (1). In other words, HIF, as its name appropriate says, is a transcription factor which assist in promoting and increasing oxygen to hypoxic regions. By the way, William Kaelin Jr., Peter J. Ratcliffe and Gregg L. Semenza were awarded the 2019 Nobel Prize in Physiology or Medicine for their work in elucidating how HIF senses and adapts cellular response to oxygen availability.
Erythropoiesis in Chronic Kidney Disease
In patients without CKD, as Hb drops, erythropoietin (EPO) production increases in an attempt to increase the Hb. This feedback regulation is either absent or is reversed in CKD patients. However, our knowledge of the underlying cause of impaired EPO production is incomplete.
What if we increase EPO “naturally” by increasing HIF? Roxadustat is a new first-in-class oral agent for the treatment of anaemia in patients with CKD and is poised to be listed on the PBS anytime soon. The others in the class include vadadustat, daprodustat and molidustat. All these oral -dustats are inhibitors of HIF prolyl hydroxylase that stimulates erythropoiesis. They also regulate iron metabolism by suppressing hepcidin which is an indirect regulator of iron absorption and utilization [2]. When hepcidin levels are suppressed, intestinal absorption of iron increases and the release of iron from storage sites are utilised.
Roxadustat is orally bioavailable with an estimated half-life of 12 h. When roxadustat was administered in doses of either 1.0 or 2.0mg/kg twice weekly or three times weekly, endogenous EPO started to increase within 4 hours of the dose and peaked at 10 hours post-dose. Endogenous EPO fall back to baseline at 24–48 h post-dose regardless of the dose given. Peak EPO increased with dose but not with frequency. At 6-week follow-up, there was a decrease in iron saturation and an increase in total iron binding capacity (TIBC) in the roxadustat group, signifying increased iron utilisation, as well as a significant decrease in hepcidin levels.
Does Roxadustat work?
In a Phase II randomised control trial, over a 12-week period, patients with CKD 3-4 with anaemia and not on dialysis, compared with placebo, patients on roxadustat were significantly more likely to increase Hb by at least 1g/dL within a 2-week period. The higher the dose of roxadustat, the higher the rates of Hb response (3). In another phase 3 trial conducted at 29 sites in China, in patients with CKD 3-5, compared with placebo, roxadustat resulted in higher Hb after 8 weeks (4).
In another study, an open label randomised study of dialysis dependent CKD patients, roxadustat administered 3 times a week in various doses was compared with parenteral EPO (5). Roxadustat was non-inferior to parenteral EPO in increasing Hb in patients with CKD and anaemia. In the subgroup of patients with elevated C-reactive protein (CRP), roxadustat resulted in greater increase in Hb and was superior to parenteral EPO in increasing Hb.
Interestingly, roxadustat, but not parenteral EPO, was associated with reduction in hepcidin levels. Hepcidin is an important mediator in iron homeostasis. Reducing hepcidin leads to improvement in iron homeostasis.
Is Roxadustat safe?
Roxadustat was well tolerated in Phase II trials, and most documented adverse events were typical for patients with CKD. Common documented side effects were diarrhoea, headache, back pain, fatigue, and increases in blood pressure and liver enzymes. Cardiovascular events were comparable in patients who received roxadustat compared to ESA. Since HIF-inhibitors effectively increase EPO and Hb, it can be hypothesised that there may be potential long-term cardiovascular and/or malignant side effects that may become apparent with longer duration trials and post-marketing analysis (6).
Roxadustat is approved in both China and Japan for the treatment of anaemia in CKD patients. I am not sure when roxadustat will be available in Australia for treatment of anaemia in patients with CKD but when it comes, we will need to know a bit more about the drug when CKD patients return from their nephrologists. My sources tell me it will be here in Australia real soon. You read about roxadustat first here at GPVoice.
References
- https://en.wikipedia.org/wiki/Hypoxia-inducible_factors
- Besarab A, Provenzano R, Hertel J, et al. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant. 2015;30(10):1665–73.
- Tadao Akizawa . Manabu Iwasaki . Tetsuro Otsuka . Michael Reusch . Toshihiro Misumi. Roxadustat Treatment of Chronic Kidney Disease-Associated Anemia in Japanese Patients Not on Dialysis: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Trial. Adv Ther (2019) 36:1438–1454
- Chen, C. Hao, X. Peng, H. Lin, et al. Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N Engl J Med 2019;381:1001-10.
- Chen, C. Hao, B.-C. Liu et al. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N Engl J Med 2019;381:1011-22.
- Kimberly Becker . Maha Saad. A New Approach to the Management of Anemia in CKD Patients: A Review on Roxadustat. Adv Ther (2017) 34:848–853
- Stauffer M et al. PLoS One. 2014.
- Thorp ML et al. Nephrology. 2009;14:240–
