22nd December 2019, Dr Chee L Khoo
There have been no new advances in acute migraine management since the introduction of the triptans in the early 1990s. There have been no advances in migraine prophylaxis since that time either. Over the last decade, there have been two emerging migraine therapies that are in advanced Phase 2 and Phase 3 trials. They are ready to hit the market anytime soon. The battle is now on between the mabs and the gepants. What are they?
Let’s look at an updated view of the pathophysiology of migraine. Calcitonin gene-related peptide (CGRP) was first discovered in 1982 (1). It is produced in both peripheral and central neurons and is a potent peptide vasodilator and can function in the transmission of nociception. During a migraine, activated meningeal nociceptors in the trigeminal ganglion release CGRP from their peripherally projecting nerve endings located within the meninges. CGRP then activates CGRP receptors located around meningeal vessels, causing vasodilation, mast cell degranulation, and plasma extravasation.
Presynaptic receptors located on trigeminal neurons regulate CGRP release. The presynaptic serotonin receptors, 5-HT1B and 5-HT1D, inhibit CGRP release. These receptors are the targets of the triptans. Triptans are also partial agonists for serotonin 5-HT1B and 5-HT1D receptors at blood vessels and nerve endings in the brain (2). See Figure 1 below. The first clinically available triptan was sumatriptan, which has been marketed since 1991. Triptans have largely replaced ergotamines, an older class of medications used to relieve migraine and cluster headaches.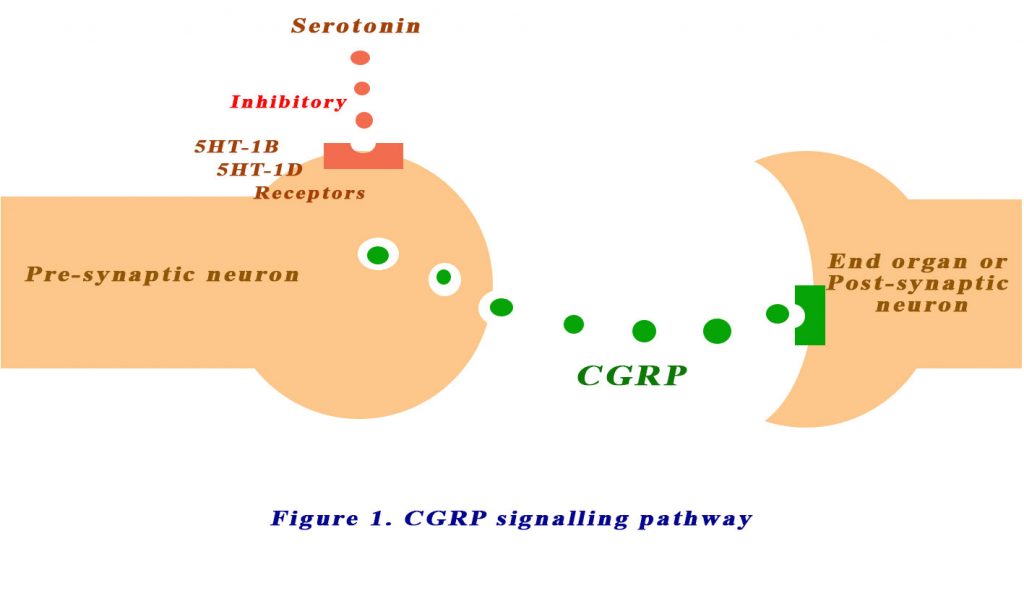
All triptans are contraindicated in patients with cardiovascular diseases (coronary spasms, symptomatic coronary artery disease, after a heart attack or stroke, uncontrolled hypertension, Raynaud’s disease, peripheral artery disease). There is also a problem of serotonin syndrome if triptans are combined with other serotonergic drugs such as ergot alkaloids, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs) or St John’s wort.
An understanding of the role of CGRP has spurned the development of various agents that block the CGRP signalling pathway:
- CGRP receptor small molecule antagonists
- Anti-CGRP receptor antibodies
- Anti-CGRP antibodies
See Figure 2 below.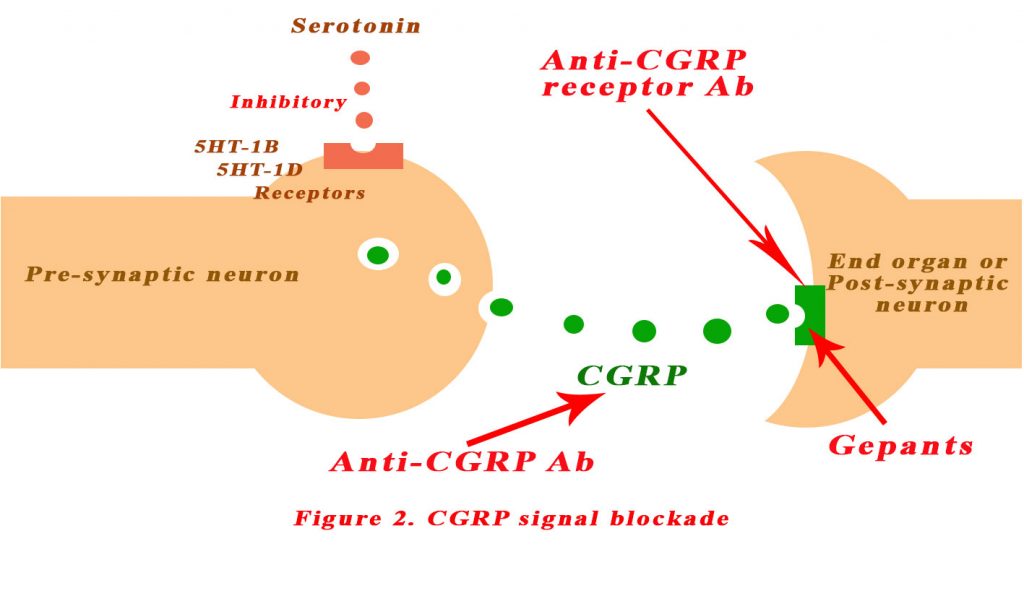
CGRP receptor small molecule antagonists
Several small molecule antagonists have been shown to block the binding of CGRP to its receptors. They are chemically un-related but they all do the same job and collectively referred as gepants. All gepants that have been tested to date are effective in patients with migraine (3-5). Olcegepant was the first to be discovered but since then others have been successfully tested – telcagepant, ubrogepant, MK-3207, BI 44370 TA and BMS-927711. However, further development of gepants has been clouded by the discovery of liver toxicity with long term use of the earlier gepants. Ubrogepant and atogepant are newly developed gepants that are chemically different from telcagepant and the other gepants. Ubrogepant has completed phase IIb testing and is being evaluated in phase III trials for acute relief of migraine. To date, results indicate that ubrogepant has good efficacy, comparable to that of triptans, with no incidence of elevated liver enzymes or other serious adverse effects (6).
The “mabs”
An alternative to small molecules for blocking CGRP transmission in patients with migraine is the use of selective monoclonal antibodies that bind to either CGRP or the CGRP receptor. The therapeutic goal of this approach is migraine prophylaxis, meaning a reduction in the number of migraine days experienced by patients who have frequent attacks.
The antibodies have a serum half life of between 20-50 days and well suited to prophylactic treatment. To date, four antibodies have been developed – one anti-CGRP receptor (erenumab) and three anti-CGRP antibodies (eptinezumab, fremanezumab and galcanezumab). These antibodies has successfully decreased the number of migraine days in episodic migraine in a number of studies (7).
Eptinezumab is administered every 3 months while the other two are monthly injections. These antibodies bind to their target site with high affinity and electivity, thus reducing the potential for unwanted, off- target effects. Furthermore, in contrast to small exogenous molecules such as the gepants, antibodies are not processed by the liver, thus avoiding the potential for liver toxicity and hepatic drug interactions.
Reduction of CGRP in theory, can cause cardiovascular pathophysiology, such as hypertension, cardiac dysfunction and episodes of coronary or cerebral ischaemia. To date, no cardiovascular adverse effects of anti- CGRP and anti- CGRP receptor antibodies have been reported in up to 6 months of phase III clinical testing.
While we are beginning to understand the CGRP signalling pathway and we have trials that demonstrate their efficacy, we still don’t actually know what causes the headaches in migraine. The triptans, gepants and antibodies have little ability to cross the blood brain barrier. So, their effects must be outside the CNS. It is now thought that the most likely targets of therapeutic CGRP antibodies and CGRP receptor blockers are within the trigeminal ganglion itself. We have much to learn about how CGRP transmission in the trigeminal ganglion contributes to migraine attacks, but the development of effective drugs that act on this target is a major milestone for patients with migraine.
Interesting times ahead.

Hot off the press!
23rd December 2019
“The U.S. Food and Drug Administration today approved Ubrelvy (ubrogepant) tablets for the acute (immediate) treatment of migraine with or without aura (a sensory phenomenon or visual disturbance) in adults. Ubrelvy is not indicated for the preventive treatment of migraine. It is the first drug in the class of oral calcitonin gene-related peptide receptor antagonists approved for the acute treatment of migraine”
References:
- Amara, S. G., Jonas, V., Rosenfeld, M. G., Ong, E. S. & Evans, R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 298, 240–244 (1982).
- https://en.wikipedia.org/wiki/Triptan (accessed 22/12/2019)
- Puledda, F., Messina, R. & Goadsby, P. J. An update on migraine: current understanding and future directions. J. Neurol. 264, 2031–2039 (2017).
- Edvinsson, L., Villalon, C. M. & MaassenVanDenBrink, A. Basic mechanisms of migraine and its acute treatment. Pharmacol. Ther. 136, 319–333 (2012).
- Edvinsson, L. & Petersen, K. A. CGRP- receptor antagonism in migraine treatment. CNS Neurol. Disord. Drug Targets 6, 240–246 (2007).
- Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the Treatment of Migraine. N Engl J Med. 2019;381(23):2230–2241. doi:10.1056/NEJMoa1813049
- Edvinsson, L., Haanes, K.A., Warfvinge, K. et al. CGRP as the target of new migraine therapies — successful translation from bench to clinic. Nat Rev Neurol 14, 338–350 (2018) doi:10.1038/s41582-018-0003-1
