5th April 2021, Dr Chee L Khoo
Last week we explored the association of thromboembolism following vaccination with AstraZeneca (AZ) vaccine. This adverse event was first reported in two patients in Austria which is then followed by more reports in Germany and the rest of Europe. One man in Victoria was recently reported to have similar adverse effects following the AZ vaccine. I managed to track down the details of initial 9 cases in Austria and Germany. I am indebted to my good friend Dr John McKenzie from Victoria who pointed me to the article. Despite the Australian Technical Advisory Group on Immunisation (ATAGI) saying that the benefits outweigh the risk of receiving the AZ vaccine, this issue will be a major discussion point between the patient and the GP when they present for vaccination. We better be equipped with the facts.
The first Austrian Case (Index case)
A previously healthy 49-year-old nurse received her first dose of AZ vaccine in mid-February 2021 (day 0). She had minor fatigue, myalgia and headache over the next few days. However, on day 5, she developed chills, fever, nausea and epigastric discomfort, and was admitted to a local hospital on day 10. The platelet count was 18 x10*9/L and d-Dimer was 35.2 mg/l (reference range, < 0.5) suggesting a thromboembolic process evolving. Her INR was 1.4 on admission but did not go up thereafter. All other blood tests except GGT (141) and C-reactive protein (8.8) were normal. Her Covid-19 nasopharyngeal swab was negative.
CTs showed portal vein thrombosis and peripheral pulmonary emboli. She received a platelet concentrate and was transferred to a tertiary hospital. Physical examination was unremarkable except for moderate epigastric tenderness on palpation. She received intravenous antibiotics, analgesia, and 4,000 U of low-molecular-weight heparin given subcutaneously.
Abdominal pain worsened and repeat CT imaging showed progression of portal vein thrombosis to include the splenic and upper mesenteric veins. In addition, small thrombi were visualized in the infrarenal aorta and both iliac arteries. Low-dose intravenous unfractionated heparin (500 IU/h) was begun but stopped shortly thereafter because of sudden onset of tachycardia and concern for gastrointestinal bleeding. Further CT revealed diffuse gastrointestinal bleeding with reduced perfusion of intestinal wall and pancreas involved by splanchnic vein thrombosis, and ascites. She received red blood cell and platelet transfusions, prothrombin complex concentrates, and recombinant factor VIIa but tragically died on day 11.
The other cases
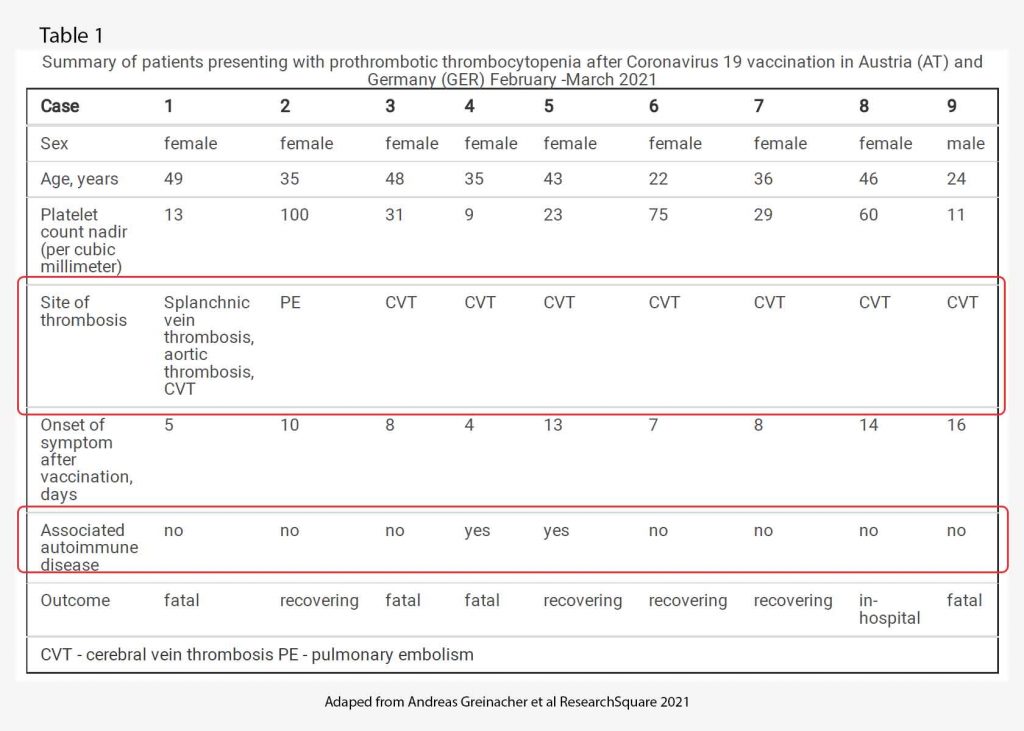
By March 15th, 2021, an additional 8 patients were identified who developed one or more thrombotic complications beginning four to 16 days following vaccination with AZ vaccine. Characteristics of all 9 patients are presented in Table 1. Of note, of the 9 patients, only 1 was male. All patients were under 50 years old. Only 2 patients had underlying autoimmune disease. All but one had cerebral venous sinus thrombosis. 4 patients died, 4 patients are recovering and one is still in hospital. The range of time of onset of symptoms after vaccination is 5-16 days.
Why
As was mentioned last week in the article, the thromboembolic events are not your usual DVTs and PEs. The clinical picture of patients with moderate to severe thrombocytopenia and thrombotic complications at unusual sites beginning approximately one week after vaccination against AZ vaccine suggests a disorder clinically resembling heparin-induced thrombocytopenia (HIT). So, what is HIT again?
Heparin-induced thrombocytopenia
As usual, let’s revise how heparin works, why HIT occurs and how the vaccine might be related to HIT. Heparin is an anticoagulant and works by binding and activating anti-thrombin III (ATIII). Activated ATIII then blocks the action of thrombin, factor Xa and other proteases leading to the anticoagulant effect.
Platelet factor 4 (PF4) is a cytokine released by activated platelets during platelet aggregation. It is meant to promote coagulation by moderating the effects of molecules like heparin. It has a role in inflammation and wound repair. In some people, the administration of heparin lead to the development of a heparin-PF4 complex when heparin binds to PF4. IgG antibodies against this complex is then developed usually after about 5 days.
The tail of this Ab-Heparin-PF4 complex binds onto the FcγIIa receptor on the surface of the platelets. This in turn activates the platelets which releases more microparticles (including PF4) leading to clot formation. The platelet count falls leading to thrombocytopenia.
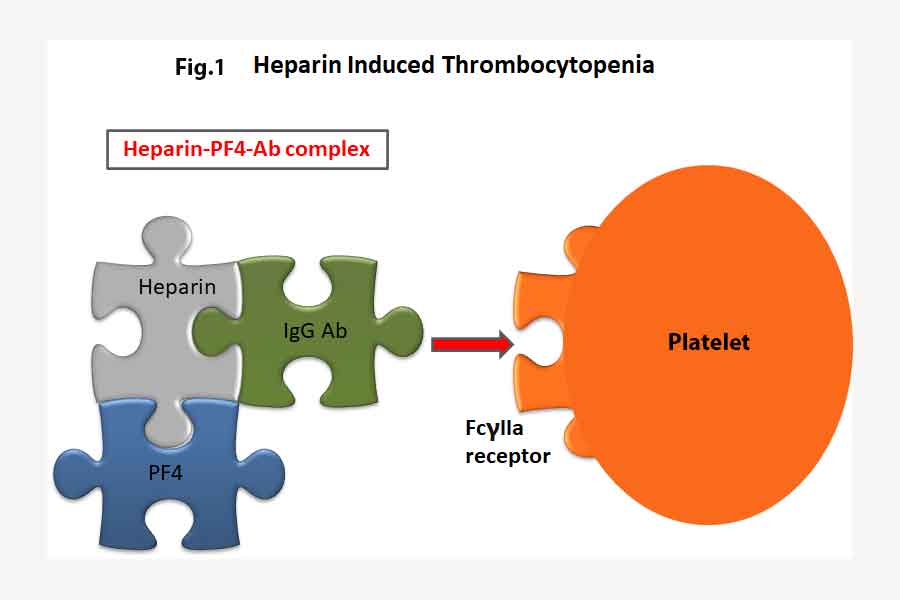
Thus, the administration of heparin to effect anti-coagulation ends up with the opposite effect in patients who developed HIT. The heparin forms a complex with PF4 and the HIT antibodies which in turn activates platelets and causes more clots.
It has been recognized that triggers other than heparin can rarely cause a disorder that strongly resembles heparin-induced thrombocytopenia on both clinical and serological grounds, including certain polyanionic drugs (e.g., pentosan polysulfate (2), antiangiogenic agent PI-88 (3), hypersulfated chondroitin sulfate (3), infections (viral, bacterial)(4), or knee replacement surgery (5,6).
Serological studies using sera from four patients who developed thrombocytopenia and thrombosis (three of them at unusual sites) following vaccination showed strong reactivity in anti-PF4/heparin enzyme-immunoassay, and also showed strong positive testing for platelet-activating antibodies.
AZ vaccine and HIT – what is the connection?
It appears that the platelet-activating antibodies induced by vaccination bind to non-complexed PF4 alone, also noted in some sera from patients with heparin-induced thrombocytopenia (7). Whether these antibodies are autoantibodies against PF4 induced by the strong inflammatory stimulus of vaccination or if the vaccine itself triggers the formation of platelet activating antibodies cannot be distinguished by this study. Enhanced reactivity of the sera in vitro in the presence of AZ vaccine could be explained by direct binding of the virus to platelets. Adenovirus binds to platelets and can cause platelet pre-activation.
The authors recommended therapy with non-heparin anticoagulants, such as those direct oral anticoagulants (rivaroxaban, apixaban). Direct oral anticoagulants (DOAC) are widely used for treatment of thrombosis in general and have also been recommended for treatment of heparin-induced thrombocytopenia. They further suggest we suggest to name this entity vaccine induced prothrombotic immune thrombocytopenia (VIPIT) to avoid confusion with heparin-induced thrombocytopenia (HIT).
What should we look for after vaccination?
- New onset severe, persistent headache or abdominal pain
- Onset between 4-20 days post vaccination
- Other features of raised intracranial pressure – vomiting, confusion
- Thrombocytopenia
- Elevated D-Dimer
This is what is on the ATAGI website (8) – last updated on 4th April:
“Rare cases of a serious unusual condition of blood clots (thrombosis) associated with low blood platelet levels (thrombocytopenia) occurring in the brain or abdomen have been reported following the COVID-19 Vaccine AstraZeneca. These cases have been rare and mostly reported overseas. One case has been reported in Australia on 1 April 2020 following administration of approximately 400,000 doses of COVID-19 Vaccine AstraZeneca. It is not yet certain if this condition is caused by the vaccine. This is still being investigated. People should discuss this information when considering the benefits and risks of COVID-19 vaccination with their immunisation provider.”
It goes on to say that “The combination of several large clinical trials involving around 57,000 people confirmed COVID-19 Vaccine AstraZeneca to be safe and effective”.
They suggest that patients discuss the issue with the immunisation provider which in most cases is and should be the GP. You better be prepared.
References:
- Andreas Greinacher, Thomas Thiele, Theodore E. Warkentin, Karin Weisser, Paul Kyrle, Sabine Eichinger. A Prothrombotic Thrombocytopenic Disorder Resembling Heparin-Induced Thrombocytopenia Following Coronavirus-19 Vaccination. Research Square www.researchsquare.com/article/rs-362354/v1. Accessed 4/4/2021.
- Tardy-Poncet B, Tardy B, Grelac F, et al. Pentosan polysulfate-induced thrombocytopenia and thrombosis. Am J Hematol 1994;45(3):252–7. DOI: 10.1002/ajh.2830450312.
- Rosenthal MA, Rischin D, McArthur G, et al. Treatment with the novel anti-angiogenic agent PI-88 is associated with immune-mediated thrombocytopenia. Ann Oncol 2002;13(5):770–6. DOI: 10.1093/annonc/mdf117.
- Warkentin TE, Makris M, Jay RM, Kelton JG. A spontaneous prothrombotic disorder resembling heparin-induced thrombocytopenia. Am J Med 2008;121(7):632–6. DOI:10.1016/j.amjmed.2008.03.012.
- Jay RM, Warkentin TE. Fatal heparin-induced thrombocytopenia (HIT) during warfarin thromboprophylaxis following orthopedic surgery: another example of ‘spontaneous’ HIT? J Thromb Haemost 2008;6(9):1598–600. DOI: 10.1111/j.1538-7836.2008.03040.x.
- Hwang SR, Wang Y, Weil EL, Padmanabhan A, Warkentin TE, Pruthi RK. Cerebral venous sinus thrombosis associated with spontaneous heparin-induced thrombocytopenia syndrome after total knee arthroplasty. Platelets 2020:1–5. DOI:10.1080/09537104.2020.1828574.
- Warkentin TE, Nazy I, Sheppard JI, Smith JW, Kelton JG, Arnold DM. Serotonin-release assay-negative heparin-induced thrombocytopenia. Am J Hematol 2020;95(1):38–47. DOI: 10.1002/ajh.25660.
- www.health.gov.au/sites/default/files/documents/2021/04/covid-19-vaccination-information-on-covid-19-astrazeneca-vaccine.pdf
