29th August 2022, Dr Chee L Khoo
By now, you would be very familiar with the additional cardiorenal benefits when you use an SGLT2 inhibitor (SGLT2i) in the management of hyperglycaemia in patients with type 2 diabetes (T2D). You would also know that the benefits are enjoyed by patients whether they have diabetes or not. DAPA-CKD published in September 2020 during the pandemic. If you missed the release 2 years ago, don’t worry. Since then additional sub-analysis have brought up interesting issues which makes a re-read of DAPA-CKD most relevant to primary care.
Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease (DAPA-CKD)
4304 participants with an eGFR of 25 – 75 ml/minute/1.73 m2 and a urinary albumin-to-creatinine ratio (with albumin measured in milligrams and creatinine measured in grams) of 200 – 5000 were randomised to either 10mg dapagliflozin or placebo in addition to a stable dose of an ACE inhibitor (ACEi) or ARB for at least 4 weeks before screening.
We don’t often pay that much attention to the baseline characteristics of the participants but in this case, they are very important as you will see later. At baseline, the characteristics, including medications for type 2 diabetes and kidney disease, were balanced between the dapagliflozin and placebo groups. The mean eGFR was 43.2 ml/min/1.73 m2 and notably, only 10% had eGFR > 60 ml/min/1.73 m2 and ~15% of participants had eGFR <30 ml/min/1.73 m2. Approximately, 2/3 of the participants had T2D, a little over 1/3 had pre-existing CVD and 11% had known heart failure. See Table 1.
The primary outcome was the composite of sustained ≥50% eGFR decline, ESKD, and renal or CV death. Now, the investigators were wise to lump renal and CV death together as sometimes, the cause of death in advanced renal disease can be hard to dissect out. Secondary outcomes included: 1) the composite outcome of sustained ≥50% eGFR decline, ESKD, or renal death; 2) composite of CV death or HHF; or 3) all-cause mortality.
Overall, 4289 participants (99.7%) completed the trial (i.e., were alive with follow-up data available at the trial completion visit or had died during follow-up). A total of 11 participants (0.3%) withdrew consent, and vital status was ascertained for all but 5 participants (0.1%).
The trial was discontinued after 2.4 years because of clear efficacy, on the basis of 408 primary outcome events.
The Topline results
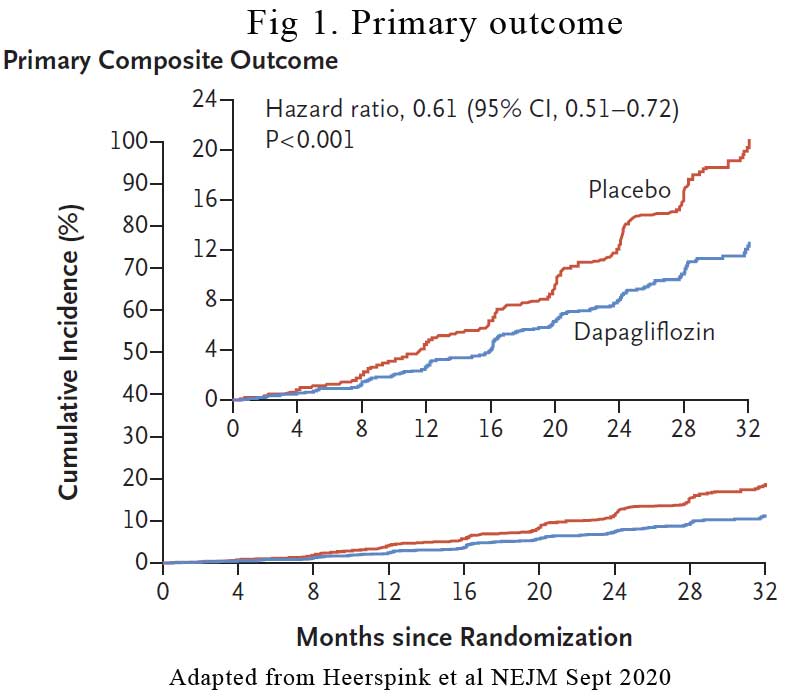
The primary outcome occurred in 197 participants (9.2%) in the dapagliflozin group vs 312 participants (14.5%) in the placebo group representing a hazard ratio of 0.61 (p<0.001). See Figure 1. The event rates for all components of the composite outcome favoured dapagliflozin. The number of participants who needed to be treated during the trial period to prevent one primary outcome event was 19. The effect of dapagliflozin on the primary outcome was generally consistent across prespecified subgroups.
In the secondary outcomes, the hazard ratio for the kidney composite of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal causes was 0.56. The hazard ratio for the composite of death from cardiovascular causes or hospitalisation for heart failure was 0.71. There were 101 deaths (4.7%) from any cause in the dapagliflozin group and 146 (6.8%) in the placebo group (hazard ratio, 0.69).
SUB-ANALYSIS
eGFR declined with Dapagliflozin
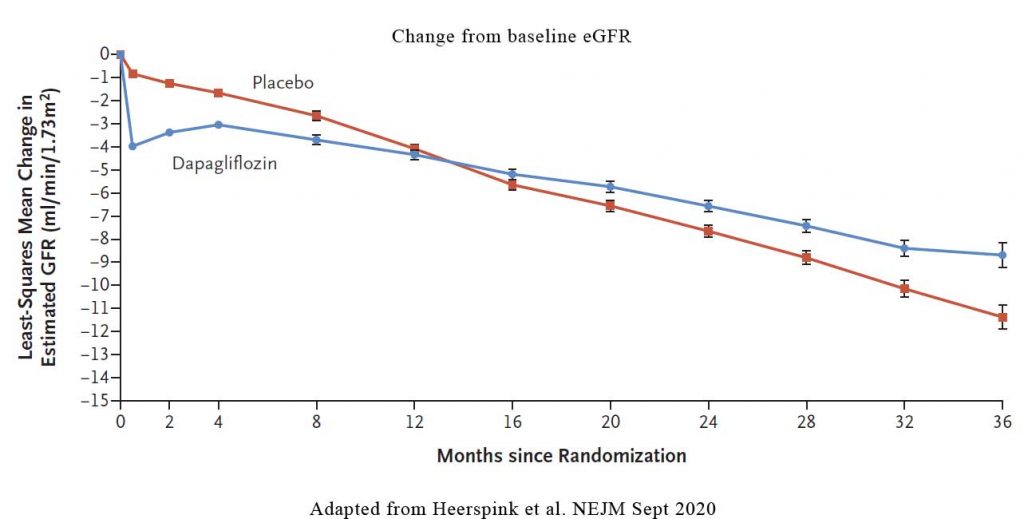
It is known that when we start patients on an SGLT2 inhibitor, in some patients, there may be a slight decline in eGFR. Indeed, in DAPA-CKD, During the first 2 weeks, there was a greater reduction in the eGFR in the dapagliflozin group than in the placebo group (–3.97±0.15 vs. –0.82±0.15 ml per minute per 1.73 m2). Thereafter, the annual change in the mean estimated GFR was smaller with dapagliflozin than with placebo. In other words, even though there may be an initial drop in eGFR in some patients, the rate of decline in eGFR with dapagliflozin is still lower than in placebo. See Figure 2.
The Details
Diabetes vs no diabetes
Interestingly, it didn’t really matter whether the participants had diabetes or not. Dapagliflozin had a lower primary outcome than placebo. In participants with type 2 diabetes, the hazard ratio for the comparison of dapagliflozin and placebo for the primary outcome was 0.64 (95% CI, 0.52 to 0.79), as compared with 0.50 (95% CI, 0.35 to 0.72) in participants without type 2 diabetes.
Outcomes according to baseline KDIGO risk categories
Not unexpectedly, patients with a higher KDIGO risk category experienced a higher event rate for kidney and cardiovascular events, in both the placebo and dapagliflozin treatment groups. The HR with dapagliflozin compared with placebo was 0.65 in patients in the very high KDIGO risk category, 0.44 among patients in the high KDIGO risk category, and 0.71 among patients in the moderately high KDIGO risk category. There were no significant differences in benefit between the KDIGO risk groups (“no heterogeneity”). In other words, dapagliflozin equally benefit all KDIGO risk groups compared with placebo.
Dapagliflozin consistently reduced the relative risks for the primary and all secondary endpoints across subgroups of UACR and eGFR (all p for interaction >0.10). Although the relative effects on the primary and kidney-specific secondary endpoint were consistent across baseline UACR subgroups, the absolute benefit on the primary and kidney-specific secondary endpoints was greater in subgroups with higher levels of albuminuria.
Participants with heart failure
11% of the participants had heart failure (HF). They were older and had more coronary disease, atrial fibrillation, and type 2 diabetes. Dapagliflozin reduced the risk of kidney failure and cardiovascular death/HF hospitalization and prolonged survival in CKD patients with or without type 2 diabetes, independently of history of HF. The proportional risk-reductions were similar in patients with and without HF for the cardiovascular death/HF hospitalization composite (HR: 0.68 vs 0.70), and all-cause death (HR: 0.56 vs 0.73)
Implications of DAPA-CKD findings
This study confirms the important role dapagliflozin play in reducing renal and cardiovascular outcomes in patients with or without diabetes, irrespective of whether heart failure is present, across all levels of chronic kidney disease. The findings of the study come on the back of similar findings with canagliflozin. We are anxiously awaiting the results of empagliflozin in the EMPEROR-KIDNEY trial which is due to report towards the end of 2022. For now, we must consider dapagliflozin in patients with diabetes who has CKD. There is no doubt that the PBS Authority indication will soon include the use of dapagliflozin in patients with CKD with or without diabetes. Watch this space.
UPDATE 1/9/2022:
Well just as I predicted, as the article went to air, Dapagliflozin is now indicated for CKD (some restrictions) under PBS authority without having to qualify for HbA1c >7.0% or having T2D at all.
References:
Heerspink HJ et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med Sept 2020;383:1436-46. DOI: 10.1056/NEJMoa2024816
