10th December 2023, A/Prof Chee L Khoo

We don’t get old and weak. Actually, if we get weak, we become old. We all see that in practice. It doesn’t matter how old our patient is. When they become weak, they slow down. They can’t walk very far and they don’t. They become weaker and weaker. They have difficulty get off a chair. They come in after the 3rd fall and sometimes, they break a bone. Sometimes, they are just bruised heavily. It’s the beginning of the end. Like most things in medicine, if we can detect patients in pre-sarcopenia, perhaps, we might be able to change the trajectory. Can we measure sarcopenia in clinical practice?
Is sarcopenia bad?
The term “saropenia” was first coined by Irwin Rosenberg in 1989 to refer to age-related loss of skeletal muscle mass. We usually think of sarcopenia mainly among older adults but it can occur earlier in life. The original operational definition of sarcopenia by European Working Group on Sarcopenia in Older People (EWGSOP) in 2010 aimed to foster advances in identifying and caring for people with sarcopenia (1). It was a major advance at that time but in 2018, they added muscle function to former definitions instead of only relying on detection of low muscle mass.
Sarcopenia is a progressive and generalised skeletal muscle disorder that is associated with increased likelihood of adverse outcomes including falls, fractures, physical disability and mortality. Muscle strength is an emerging risk factor for CVD. It is known that muscle strength is lower in people with type 2 diabetes (T2D) (2) because of an accelerated loss of muscle with age (3). The prevalence of sarcopenia in people with T2D has been reported as between 7% and 29.3%, (4) and this could potentially explain the excess CVD risk in people with T2D.
It has also been shown that amongst people with T2D, the excess CVD risk was greater in those with low versus high muscle strength (5). In an observational study, Zheng et al showed that the 10-year CVD risk was 46.2% higher in people with sarcopenia without anaemia and doubled in those with T2D and anaemia (6). However, the diagnosis of sarcopenia was based on muscle mass and the increase in risk was calculated from the Framingham score rather than actual events.
In a prospective cohort study with 11,974 White European UK Biobank participants with T2D aged 40-70 years, the association between sarcopenia and the incidence of CVD was investigated (7). Sarcopenia was defined based on the EWGSOP as either non-sarcopenic or sarcopenic. Compared with non-sarcopenia, those with sarcopenia had higher risks of CVD (HR 1.89), HF (HR 2.59), stroke (HR 1.90), and MI (HR 1.56). Those with sarcopenia had CVD incidence rates equivalent to those without sarcopenia who were 14.5 years older.
How is sarcopenia defined by the EWGSOP?
When you think about it, it is actually difficult to define what is low muscle mass. Some people are big and some people are small. Non-European are generally smaller in stature. How does one quantify what is low muscle mass in different population? Sarcopenia is considered a muscle disease (muscle failure), with low muscle strength overtaking the role of low muscle mass as a principal determinant. This change is expected to facilitate prompt identification of sarcopenia in practice. Muscle mass and muscle quality are technically difficult to measure accurately.
MRI and CT are considered to be gold standards for non-invasive assessment of muscle quantity/mass. Dual-energy X-ray absorptiometry (DXA) is a more widely available instrument to determine muscle quantity non-invasively, but different DXA instrument brands do not give consistent results. However, these tools are not commonly used in primary care.
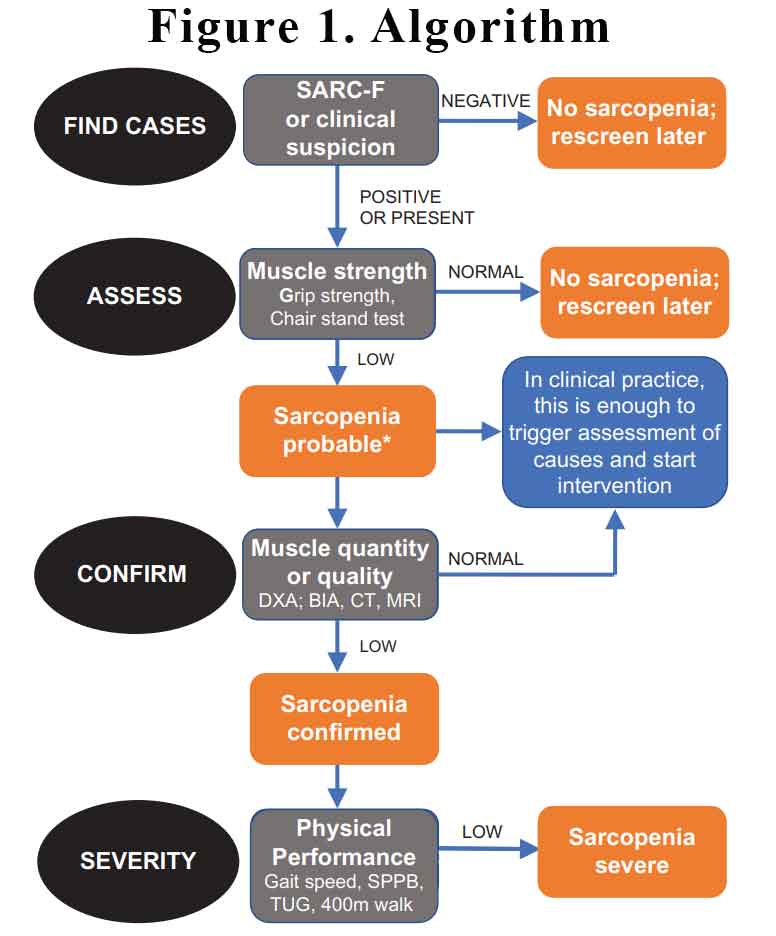
2018 EWGSOP2 operational definition of sarcopenia
Criterion
(1) Low muscle strength
(2) Low muscle quantity or quality
(3) Low physical performance
Probable sarcopenia is identified by Criterion 1.
Diagnosis is confirmed by additional documentation of Criterion 2.
If Criteria 1, 2 and 3 are all met, sarcopenia is considered severe.
How can we screen for sarcopenia?
The SARC-F questionnaire is a screening tool that can be rapidly implemented by clinicians to identify probable sarcopenic patients. The questionnaire screens patients for self-reported signs suggestive of sarcopenia, which include deficiencies in strength, walking, rising from a chair, climbing stairs, and experiencing falls. SARC-F has a low-to-moderate sensitivity and a very high specificity to predict low muscle strength. As such, SARC-F will mostly detect severe cases. Download an example of the SARC-F questionnaire here.
How can we measure sarcopenia?
In clinical practice, we can use the SARC-F questionnaire to find individuals with probable sarcopenia. There are simple tests we can perform to assess for sarcopenia:
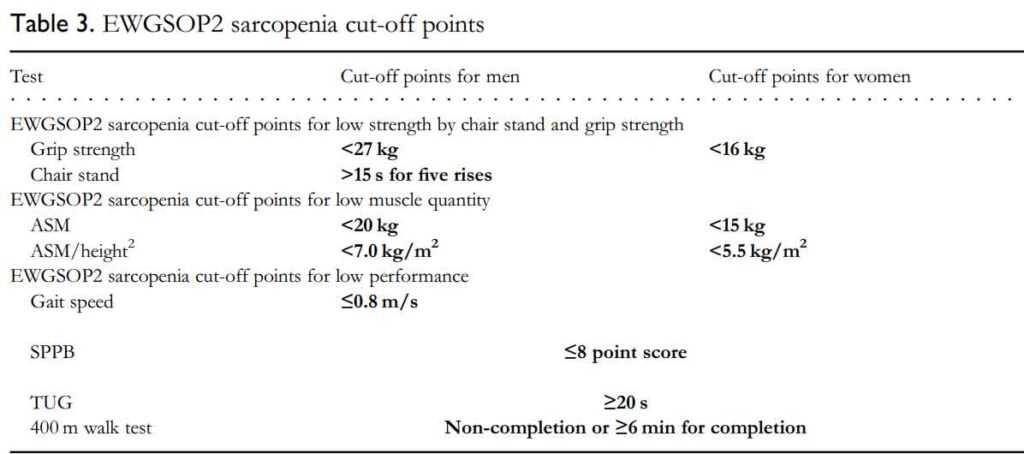
Grip strength
Low grip strength is a powerful predictor of poor patient outcomes such as longer hospital stays, increased functional limitations, poor health-related quality of life and death. Accurate measurement of grip strength requires use of a calibrated handheld dynamometer under well-defined test conditions with interpretive data from appropriate reference populations.
Chair stand
The chair stand test measures the amount of time needed for a patient to rise five times from a seated position without using his or her arms.
Gait speed
A commonly used gait speed test is called the 4-m usual walking speed test, with speed measured either manually with a stopwatch or instrumentally with an electronic device to measure gait timing.
Short Physical Performance Battery (SPPB)
The SPPB is an objective measurement instrument of balance, lower extremity strength, and functional capacity in older adults (>65 years of age). The test includes three different domains (walking, sit-to-stand and balance) to assess functional mobility.
An example of how to do the SPPB can be found here.
Timed up and Go (TUG) test
- The patient starts in a seated position
- The patient stands up upon therapist’s command: walks 3 meters, turns around, walks back to the chair and sits down.
- The time stops when the patient is seated.
An example of the full test can be found here.
400 m walk test
The 400-m walk test assesses walking ability and endurance. For this test, participants are asked to complete 20 laps of 20 m, each lap as fast as possible, and are allowed up to two rest stops during the test.
The EWGSOP2 sarcopenia cut-off points are listed below:
The EWGSOP2 have recommended that we screen for sarcopenia, assess using simple clinical tests, confirm with more investigations if appropriate and assess severity. See Figure 1.
Muscle mass and strength vary across a lifetime—generally increasing with growth in youth and young adulthood, being maintained in midlife and then decreasing with ageing. In young adulthood (up to ~40 years of age), maximal levels, which are higher in men than in women, are reached. Beyond the age of 50 years, loss of leg muscle mass (1–2% per year) and loss of strength (1.5–5% per year) have been reported. Interestingly, there is a positive association between birth weight and muscle strength, which is maintained across the life course.
While genetic and lifestyle factors can hasten muscle weakening and progression toward functional impairment and disability, interventions including nutrition and exercise training seem to slow or reverse these processes. Therefore, to prevent or delay sarcopenia, the aim is to maximise muscle in youth and young adulthood, maintain muscle in middle age and minimise loss in older age.
In summary, we preside over our patients getting older and weaker over the years we look after them. By the time they have falls and fractures or near misses, their sarcopenia is in its advanced stage. We can easily institute a screening program to identify sarcopenia in its early stages and commence treatment to slow down the progression. Remember, sarcopenia is associated with not just an increase in falls and fractures but also with increased cardiovascular mortality.
References:
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 2010; 39: 412–23.
- Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55(6):1813-1818.
- Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30(6): 1507-1512.
- Izzo A, Massimino E, Riccardi G, Della PG. A narrative review on sarcopenia in type 2 diabetes mellitus: prevalence and associated factors. Nutrients. 2021;13(1):183.
- Celis-Morales CA, Petermann F, Hui L, et al. Associations between diabetes and both cardiovascular disease and all-cause mortality are modified by grip strength: evidence from UK biobank, a prospective population-based cohort study. Diabetes Care. 2017;40(12):1710-1718.
- Zeng F, Huang L, Zhang Y, et al. Additive effect of sarcopenia and anemia on the 10-year risk of cardiovascular disease in patients with type 2 diabetes. J Diabetes Res. 2022;2022:2202511.
- Boonpor J, et al In people with type 2 diabetes, sarcopenia is associated with the incidence of cardiovascular disease: A prospective cohort study from the UK Biobank. Diabetes Obes Metab. 2023;1–8.
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jul 1;48(4):601. doi: 10.1093/ageing/afz046. Erratum for: Age Ageing. 2019 Jan 1;48(1):16-31.
