24th February 2024, A/Prof Chee L Khoo

We all know how important it is for BP control in prevention cardiovascular, cerebrovascular and renal complications. We are also aware of the difficulty is improving medication adherence. We have a broad range of effective BP lowering medications covering diverse classes of medications. Anti-hypertensives work only if our patients take them regularly. Furthermore, even patients who appear to have well-controlled hypertension during periodic office visits may experience significant between-visit variability, which is associated with residual risk for cardiovascular events (1-6). Although a broad range of patient and clinician factors contribute to inadequate BP control, inconsistent adherence to complex, multidrug oral treatment regimens that require daily dosing may be an important driver. What if we have a long acting injectable anti-hypertensive that doesn’t depend on our patient remembering to take their medication on time? Would that improve BP control?
Global estimates suggest that up to 80% of patients with hypertension do not meet guideline-recommended blood pressure (BP) targets (7,8). Every 10‐mm Hg reduction in systolic BP leads to a 13% reduction in all‐cause mortality, while significantly reducing the risk of major cardiovascular events (20%), coronary heart disease (17%), stroke (27%), and heart failure (28%) (9). We have a problem in primary care. As many things can affect day to day BP readings, we may sometimes have to sacrifice slightly higher readings when patients come in with illness, with pain or on a bad day. I know we shouldn’t succumb to excuses “why” the BP might be high today but we also have to be careful about postural hypotension.
Zilebesiran is a small interfering RNA (siRNA) that is designed to achieve specific reduction in hepatic angiotensinogen messenger RNA (mRNA) levels, thereby reducing the hepatic production of angiotensinogen, a therapeutic target for hypertension. Of course, angiotensinogen is the predominant precursor for angiotensin peptides and a key regulator of systemic BP. By targeting angiotensinogen (instead of angiotensin), it may theoretically limit compensatory angiotensin activation associated with angiotensin-converting–enzyme inhibition or angiotensin-receptor blockade (10-12). By targeting hepatic RNA, extrahepatic angiotensinogen expression may be preserved, limiting off-target effects in the kidney and other tissues.
In a small Phase 1 study, single doses of zilebesiran (≥200 mg) were associated with decreases in systolic blood pressure (>10 mm Hg) and diastolic blood pressure (>5 mm Hg) by week 8 (13). These changes were consistent throughout the diurnal cycle and were sustained at 24 weeks (). The results of Phase 2 study has just published.
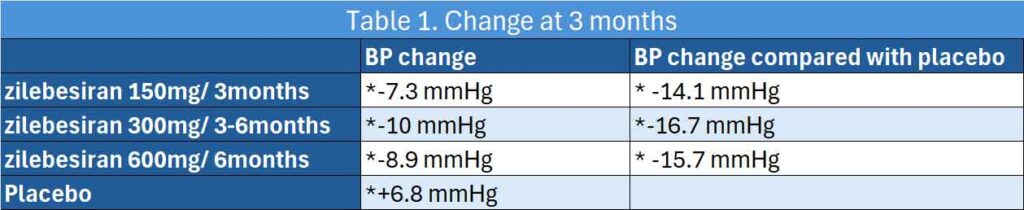
394 Adults with mild to moderate hypertension, defined as daytime mean ambulatory systolic BP (SBP) of 135 to 160 mm Hg following antihypertensive washout, were randomised to either zilebesiran (150, 300, or 600 mg once every 6 months or 300 mg once every 3 months) or placebo every three months (14). At 3 months, 24-hour mean ambulatory SBP changes from baseline were −7.3 mm Hg with zilebesiran 150 mg, once every 6 months; −10.0 mm Hg with zilebesiran 300 mg, once every 3 months or every 6 months; −8.9 mm Hg with zilebesiran 600 mg, once every 6 months; and 6.8 mm Hg with placebo.
LSM differences vs placebo in change from baseline to month 3 were −14.1 mm Hg with zilebesiran, 150 mg, once every 6 months; −16.7 mm Hg with zilebesiran, 300 mg, once every 3 months or every 6 months; and −15.7 mm Hg with zilebesiran, 600 mg, once every 6 months.
See Table 1.
Safety issues
Serious AEs were reported in 5 patients (6.7%) in the placebo group and 11 patients (3.6%) in the zilebesiran groups, including 1 death due to cardiopulmonary arrest 5 days after treatment with zilebesiran, 300 mg, none of which were considered by the investigators to be related to the study drug.
Four patients had drug-related AEs leading to an investigator decision to discontinue further study drug dosing during the double-blind period (orthostatic hypotension [n = 2], BP elevation [n = 1], and injection site reaction [n = 1]). Clinically relevant AEs of acute kidney failure were reported in 4 patients (1.3%) receiving zilebesiran vs 0 receiving placebo, hepatic AEs were reported in 9 patients (3.0%) receiving zilebesiran vs 1 (1.3%) receiving placebo, hypotension was reported in 13 (4.3%) receiving zilebesiran vs 1 (1.3%) receiving placebo, and hyperkalemia was reported in 19 (6.3%) patients receiving zilebesiran vs 2 (2.7%) receiving placebo.
Over all, over 6 months, 60.9% of patients receiving zilebesiran had adverse events vs 50.7% patients receiving placebo and 3.6% had serious adverse events vs 6.7% receiving placebo. Nonserious drug-related adverse events occurred in 16.9% of zilebesiran-treated patients (principally injection site reactions and mild hyperkalemia) and 8.0% of placebo-treated patients.
Wow. It’s not just the degree of reduction in BP patients with mild to moderate hypertension but the sustained nature of the reduction. Zilebesiran appears to provide better 24 hour control. It can be administered every 3 months and possibly every 6 months. Patients are actually quite used to injections now, whether self administered or administered by the primary care team every 3-6 months. There were reports of hypotension but the rate was low (4.3%).
It will be sometime yet before we see zilebesiran arriving in Australia but I thought this is a novel way to getting our patients better control of their BP.
References:
- Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10(3):143-155. doi:10.1038/nrcardio.2013.1
- Rothwell PM, Howard SC, Dolan E, et al; ASCOT-BPLA and MRC Trial Investigators. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9(5):469-480. doi:10.1016/S1474-4422(10)70066-1
- Verdecchia P, Angeli F, Mazzotta G, et al. Day-night dip and early-morning surge in blood pressure in hypertension: prognostic implications. Hypertension. 2012;60(1):34-42. doi:10.1161/HYPERTENSIONAHA.112.191858
- Messerli FH, Hofstetter L, Rimoldi SF, Rexhaj E, Bangalore S. Risk factor variability and cardiovascular outcome: JACC review topic of the week. J Am Coll Cardiol. 2019;73(20):2596-2603. doi:10.1016/j.jacc.2019.02.063
- Kario K, Hoshide S, Mizuno H, et al; JAMP Study Group. Nighttime blood pressure phenotype and cardiovascular prognosis: practitioner-based nationwide JAMP study. Circulation. 2020;142(19):1810-1820. doi:10.1161/CIRCULATIONAHA.120.049730
- de Havenon A, Fino NF, Johnson B, et al. Blood pressure variability and cardiovascular outcomes in patients with prior stroke: a secondary analysis of PRoFESS. Stroke. 2019;50(11):3170-3176. doi:10.1161/STROKEAHA.119.026293
- World Health Organization. Hypertension. Published March 16, 2023. Accessed December 20, 2023. https://www.who.int/news-room/fact-sheets/detail/hypertension
- Million Hearts Foundation. Estimated hypertension prevalence, treatment, and control among US adults: tables. Accessed February 7, 2024. https://millionhearts.hhs.gov/files/Estimated-Hypertension-Prevalence-tables-508.pdf
- Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta‐analysis. Lancet. 2016; 387:957–967. doi: 10.1016/S0140-6736(15)01225-8
- Morgan ES, Tami Y, Hu K, et al. Antisense inhibition of angiotensinogen with IONIS-AGT-LRx: results of phase 1 and phase 2 studies. JACC Basic Transl Sci 2021;6:485-496.
- Mullick AE, Yeh ST, Graham MJ, Engelhardt JA, Prakash TP, Crooke RM. Blood pressure lowering and safety improvements with liver angiotensinogen inhibition in models of hypertension and kidney injury. Hypertension 2017;70:566-576.
- Kahlon T, Carlisle S, Otero Mostacero D, Williams N, Trainor P, DeFilippis AP. Angiotensinogen: more than its downstream products: evidence from population studies and novel therapeutics. JACC Heart Fail 2022;10:699-713.
- Desai AS, Webb DJ, Taubel J, Casey S, Cheng Y, Robbie GJ, Foster D, Huang SA, Rhyee S, Sweetser MT, Bakris GL. Zilebesiran, an RNA Interference Therapeutic Agent for Hypertension. N Engl J Med. 2023 Jul 20;389(3):228-238. doi: 10.1056/NEJMoa2208391. PMID: 37467498.
- Bakris GL, Saxena M, Gupta A, et al. RNA Interference With Zilebesiran for Mild to Moderate Hypertension: The KARDIA-1 Randomized Clinical Trial. JAMA. Published online February 16, 2024. doi:10.1001/jama.2024.0728
