29th January 2023, Dr Chee L Khoo
We looked at the difference in definitions between non-alcoholic and metabolic-dysfunction associated fatty liver disease (so-called NAFLD vs MAFLD) in the last issue of GPVoice. There are many similarities and overlap between the two entities. At the end of the day, does it really matter we call the diseases. Since writing the article, I have diagnosed quite a few MAFLD and taken the appropriate next steps in the management. While some were just a migration across from NAFLD to MAFLD, many were newly diagnosed MAFLD. In most of them, the diagnosis led to significant changes in their management. Let’s have a look at the next steps after diagnosis of either NAFLD or MAFLD.
Before we can discuss the next steps after diagnosis, we need to quickly go back to the pathophysiological steps in steatosis.
The sequence of events
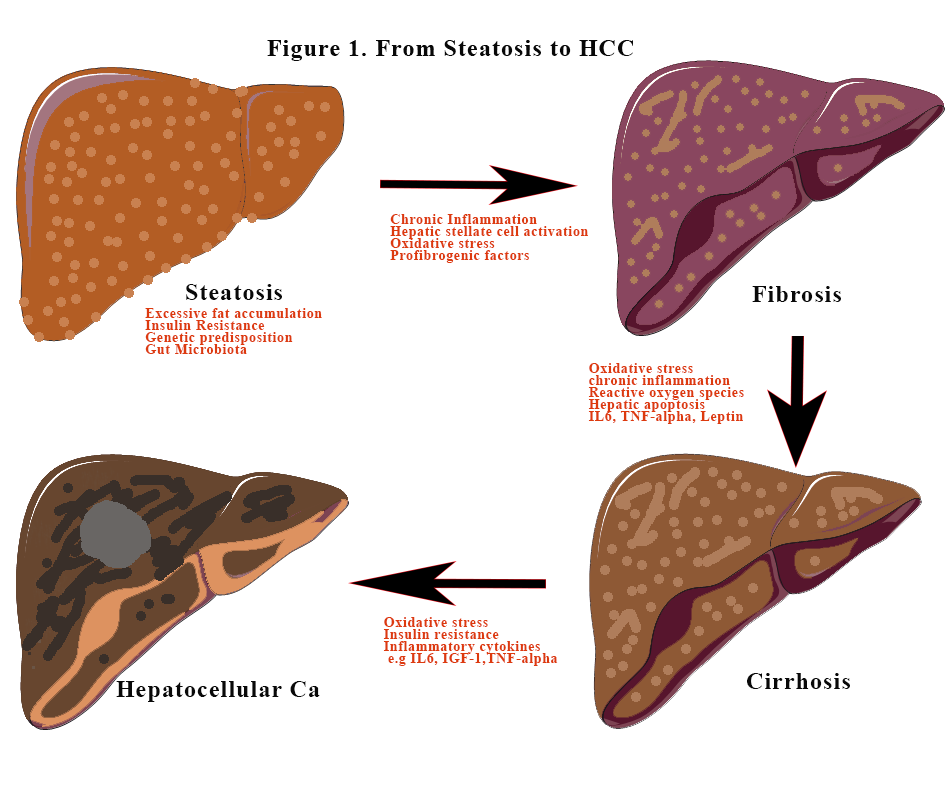
The liver complications
Excess fat accumulation and insulin resistance which feeds each other leads to increase fat storage in the liver. This is steatosis if the fat content is >5%. Inflammation is the key to further damage to hepatic tissues. Not all steatosis will lead to inflammation. Inflammation exacerbates hepatic steatosis and instigates innate inflammatory responses causing recruitment and activation of immune cells to the liver. Activation of immune cells leads to the release of numerous pro-inflammatory chemokines such as macrophage chemokines, which are responsible for the recruitment of neutrophils and monocyte-derived macrophages) and cytokines such as monocyte chemoattractant protein-1 and tumour necrosis factor-a [TNF-a]).
Insulin resistance and dyslipidaemia impair nitric acid synthesis. Nitric oxide is thought to be hepatic protective as it manages lipogenesis and it enhances beta-oxidation of fatty acid. Endotoxins produced by the “wrong” gut biota further enhances the inflammation. These gut metabolites also stimulate the secretion of tumorigenic factors. No single bacterium has been isolated but rather there is a change in the relative abundance of multiple types of microbiota including Escherichia, Prevotella, Streptococcus, Coprococcus, Faecalibacterium, and Ruminococcus.[1]. Bone-marrow derived monocytes are recruited during the inflammatory process and are also critical for MAFLD progression.
In MAFLD, hepatic stellate cells (HSC) activation releases pro-fibrogenic and inflammatory cytokines and mediators that drive fibrosis [2]. The majority of MAFLD patients have simple steatosis with little or no fibrosis. In a minority (5%–10%), the disease will evolve to steatohepatitis with subsequent fibrosis, cirrhosis, and in some, hepatocellular carcinoma (HCC).
MAFLD patients with advanced fibrosis and cirrhosis are at increased risk of HCC, though HCC can also arise in the absence of advanced fibrosis (3). The increasing trend of MAFLD-induced HCC mirrors the increase in the incidence of obesity and metabolic syndrome (4-6). HCC incidence is 12 times higher in patients with severe steatohepatitis than in the general MAFLD population and most cases occur in older males with the metabolic syndrome.[3]
The Non-liver Complications
CVD shared many risk factors with MAFLD, including diabetes, abnormal lipid metabolism and hypertension. So, it is not surprising that patients diagnosed with MAFLD has a significantly higher risk of cardiovascular events. In a meta-analysis, Wen Wen et al investigated the risk of CVD incidence or CVD mortality in patients diagnosed with MAFLD (7). 10 studies were included in the study. The main CVDs included coronary heart disease, myocardial infarction, heart failure, cerebrovascular disease, stroke, and transient ischemic attack. Overall, the incidence of CVD or CVD mortality was 1.95 times higher in the MAFLD group than in the control group. The incidence of CVD and CVD mortality was 2.26 times and 1.57 times higher in the MAFLD group than in the control group, respectively.
What about MAFLD and CKD?
CVD and CKD share many risk factors including T2D, hypertension, obesity, dyslipidaemia, and insulin resistance. There are similarities in their pathophysiologic mechanisms as inflammation, oxidative stress, fibrosis, and gut dysbiosis are highly prevalent in these pathologic states. Further, there is evidence which suggests a genetic predisposition to MAFLD due to gene polymorphisms, such as the PNPLA3 rs738409 G allele polymorphism, which may also predispose the same patients to renal dysfunction.
In an analysis of approximately 270,000 individuals that underwent (Korean) National Health Insurance Service health examinations, MAFLD was associated with an increased risk of incident CKD compared to non-metabolic NAFLD (adjusted hazard ratio 1.18) (8). In a Chinese cohort of 6873 participants with a 4.6-year follow-up, the investigators noted a higher risk of CKD in MAFLD subjects (hazard ratio 1.71) (9).
Because both MAFLD and CKD’s pathophysiology revolves around inflammation and fibrosis, the association between liver fibrosis and CKD is even greater. Ciardullo et al, in their meta-analysis of seven cross-sectional studies, detected an significant association of non-invasively assessed liver fibrosis with increased urinary albumin-to-creatinine ratio (hazard ratio 1.98) and incident CKD (hazard ratio 2.49) (10). Liver fibrosis is associated with early kidney dysfunction. In the meta-analysis by Musso et al., advanced fibrosis and inflammation were associated with more severe kidney dysfunction than hepatic steatosis itself. They concluded that the more severe the liver histology, the more severe the CKD (11).
The alternative causes of MAFLD
While MAFLD allows us to include steatosis irrespective of causes, there are other alternative causes of steatosis apart from those related to insulin resistance, dyslipidaemia and obesity. The so-called alternative causes (rather than secondary causes) include conditions such as: medications (corticosteroids, valproic acid, tamoxifen, methotrexate, amiodarone and some immunotherapeutic agents), coeliac disease, starvation, total parenteral nutrition, severe surgical weight loss or disorders of lipid metabolism (abetalipoproteinemia, hypobetalipoproteinemia, lysosomal acid lipase deficiency, familial combined hyperlipidaemia, lipodystrophy, Weber–Christian syndrome, glycogen storage disease, Wilson disease) (12).
In summary, the narrow definition of NAFLD over the years has allowed many with fatty liver disease to be sidelined and trivialised to some extent. With the adoption of MAFLD as an umbrella diagnosis of dysmetabolism in various organs, it is important to think of MAFLD as a liver manifestation of insulin resistance and metabolic syndrome. I am diagnosing more MALFD in my practice. Every time I see a patient who has features of metabolic syndrome including T2D, dyslipidaemia with or without obesity, a liver ultrasound is ordered to confirm steatosis if that has not been done.
If ultrasound has already shown steatosis, it is vital to assess for fibrosis. The more severe the fibrosis is, the higher the risk of CVD and CKD and the risk of HCC. The use of the various blood markers (FIB4, MAFLD scores) helps predict the presence of fibrosis. Sheer elastography is increasingly used as a screening tool as Fibroscan® is difficult to source. We shall explore the assessment of fibrosis in our next issue on MAFLD.
The next step is to screen for CKD and assess CV risk which will lead to a reappraisal of lipid targets. Often, a coronary artery calcium score is necessary to further inform us of the real cardiovascular risk.
References:
- Li F, Ye J, Shao C, Zhong B. Compositional alterations of gut microbiota in nonalcoholic fatty liver disease patients: a systematic review and Meta-analysis. Lipids Health Dis 2021;20:22. doi: 10.1186/s12944-021-01440-w.
- Ramos-Lopez O, Martinez-Lopez E, Roman S, Fierro NA, Panduro A. Genetic, metabolic and environmental factors involved in the development of liver cirrhosis in Mexico. World J Gastroenterol 2015;21:11552–11566. doi: 10.3748/wjg.v21.i41.11552.
- Yu J. Obesity, Fatty Liver and Liver Cancer. Singapore: Springer; 2018. 157.
- Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors, and prevention. Nat Rev Gastroenterol Hepatol 2021;18:223–238. doi:10.1038/s41575-020-00381-6.
- Sagnelli E, Macera M, Russo A, Coppola N, Sagnelli C. Epidemiological and etiological variations in hepatocellular carcinoma. Infection 2020;48:7–17. doi: 10.1007/s15010-019-01345-y.
- Oda K, Uto H, Mawatari S, Ido A. Clinical features of hepatocellular carcinoma associated with nonalcoholic fatty liver disease: a review of human studies. Clin J Gastroenterol 2015;8:1–9. doi: 10.1007/s12328-014-0548-5.
- Wen W, Li H, Wang C, Chen C, Tang J, Zhou M, Hong X, Cheng Y, Wu Q, Zhang X, Feng Z, Wang M. Metabolic dysfunction-associated fatty liver disease and cardiovascular disease: A meta-analysis. Front Endocrinol (Lausanne). 2022 Sep 16;13:934225. doi: 10.3389/fendo.2022.934225.
- 10. Jung CY, Koh HB, Park KH, Joo YS, Kim HW, Ahn SH, Park JT, Kim SU. Metabolic dysfunction-associated fatty liver disease and risk of incident chronic kidney disease: A nationwide cohort study. Diabetes Metab. 2022;48:101344.
- Liang, Y.; Chen, H.; Liu, Y.; Hou, X.;Wei, L.; Bao, Y.; Yang, C.; Zong, G.;Wu, J.; Jia,W. Association ofMAFLDWith Diabetes, Chronic Kidney Disease, and Cardiovascular Disease: A 4.6-Year Cohort Study in China. J. Clin. Endocrinol. Metab. 2022, 107, 88–97.
- Ciardullo S, Perseghin G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int 2021;41(6):1290–1293. doi:10.1111/liv.14828.
- Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med 2014;11(7):e1001680. doi:10.1371/journal.pmed.1001680.
- Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020 Jul;73(1):202-209. doi: 10.1016/j.jhep.2020.03.039.
