11th March 2023, Dr Chee L Khoo
Where did it come from? You would have fielded questions from more than a few patients about this new wonder cholesterol lowering drug that is coming to Australia “soon”. That’s what one of the current affairs media was hinting during the week. Of course, they make it sound as if everyone on statins is crying with muscle aches and pains. And this wonder drug will solve all our problems when it comes to cholesterol lowering therapy. Of course, they are talking about bempedoic acid. “When is it coming, doc?”. Do we know what bempedoic acid is? How does it compare with statins in cholesterol lowering? How does it compare with statins in cardiovascular outcomes?
Statin-intolerance
Yes, the media makes looks like “most” patients on statins have adverse musculoskeletal side effects be that aches, pains, fatigues or weakness. Between 7-29% of patients report adverse musculoskeletal effects that prevent them from using statins or limit their ability to receive guideline-recommended doses to get their LDL-C to target (1-3). Of course, ceasing the statin is associated with a higher cardiovascular risk. So, a drug that lower cholesterol levels without the musculoskeletal side effects would be ideal.
What is bempedoic acid?
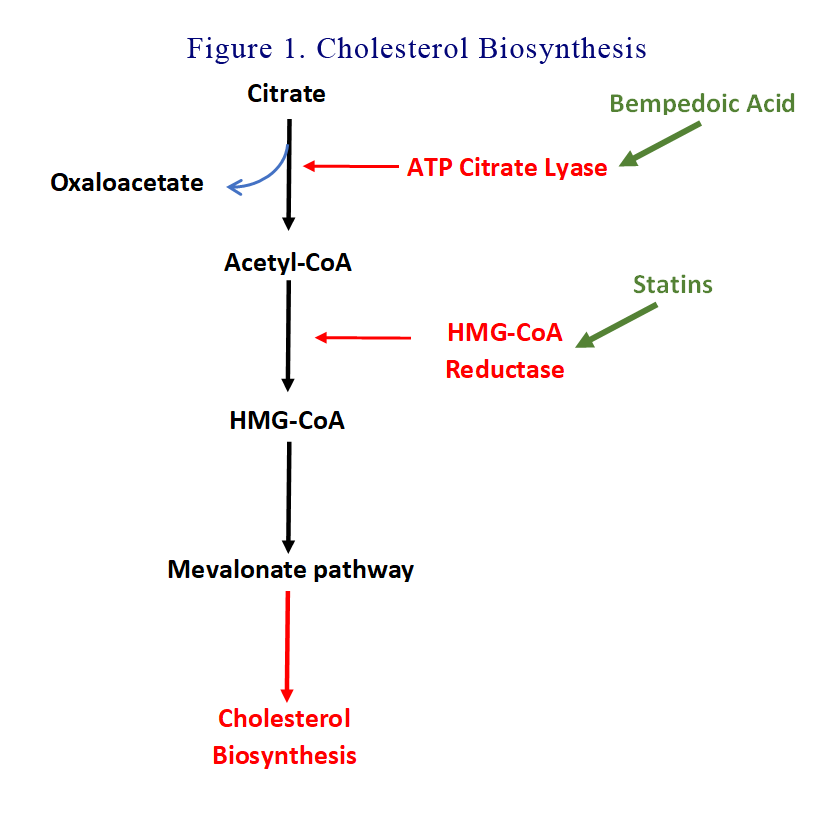
We better revise the cholesterol biosynthesis pathway. Citrate is split into oxaloacetate and Acetyl-CoA by ATP Citrate lyase (ACL). Acetyl-CoA is then reduced to HMG-CoA by HMG-CoA reductase. HMG-CoA is then funnelled into the Mevalonate pathway to synthesise cholesterol. You will recall that statins are HMG coenzyme A reductase inhibitors.
Bempedoic acid goes upstream and inhibit ACL. Bempedoic acid is a prodrug that is activated in the liver and not in most peripheral tissues, including skeletal muscle, a factor that may reduce the potential for adverse effects on muscles.
See Figure 1.
How efficacious is bempedoic acid?
Bempedoic acid has been shown in a number of studies to reduce LDL-C by 17-28% (9-12). This prompted approval by the US FDA and European Medicines Agency (EMA) in 2020. Those were primarily efficacy trials demonstrating how efficacious the agent was. Reduction in LDL-C in all cholesterol lowering trials is associated with reduction in cardiovascular outcomes. Only problem with the earlier bempedoic acid trials was that the effect on cardiovascular outcomes were not demonstrated.
What is the effect on cardiovascular outcomes?
In the most recent CLEAR (Cholesterol Lowering via Bempedoic Acid [ECT1002], an ACL-Inhibiting Regimen) Outcomes trial, Nissen et al explored the effects of bempedoic acid on adverse cardiovascular events in a mixed population of patients for whom primary or secondary prevention is clinically indicated but who were unable or unwilling to take guideline-recommended doses of statins (8).
Patients aged between 18-85 years were recruited if they either a previous cardiovascular event (secondary-prevention patients) or clinical features that placed them at high risk for a cardiovascular event (primary-prevention patients). Patients were generally either not able to tolerate statins or unwilling to start on a statin because of previous adverse effects. There were some who were on a very small dose of statins. Other lipid-lowering therapies were permitted, such as ezetimibe, niacin, bile acid resins, fibrates, or proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors, administered as monotherapy or in combinations.
The primary end point was a 4-component composite of major adverse cardiovascular events, defined as death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularisation. Secondary end points, also assessed in a time-to-first-event analysis and tested in a hierarchical order, included a three-component composite of death from cardiovascular causes, nonfatal stroke, or nonfatal myocardial infarction; fatal or nonfatal myocardial infarction; coronary revascularisation; fatal or nonfatal stroke; death from cardiovascular causes; and death from any cause.
The mean age of the cohort was 65.5 years. 46% of the participants had diabetes and 70% had previous cardiovascular event. 23% were taking a statin. 11% were on ezetimibe. The mean LDL-C was 3.6 mmol/L. The mean HDL-C was 1.3 mmol/L. Patients were followed up for 40.6 months on average.
The mean LDL-C after 6 months of treatment with bempedoic acid was 2.77 mmol/L compared with 3.52 mmol/L with representing a reduction of 21%. By the end of the trial, the reduction in LDL-C with bempedoic acid was 19.4%.
A primary end-point event (death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularisation) occurred in 819 patients (11.7%) in the bempedoic acid group and in 927 patients (13.3%) in the placebo group representing a hazard ratio 0.87.
Death from cardiovascular causes, nonfatal stroke, or nonfatal myocardial infarction (the first key secondary end point) occurred in 575 patients (8.2%) in the bempedoic acid group and in 663 patients (9.5%) in the placebo group (hazard ratio, 0.85). Fatal or nonfatal myocardial infarction (the second key secondary end point) occurred in 261 patients (3.7%) in the bempedoic acid group and in 334 patients (4.8%) in the placebo group (hazard ratio, 0.77). Coronary revascularisation (the third key secondary end point) occurred in 435 patients (6.2%) in the bempedoic acid group and in 529 patients (7.6%) in the placebo group (hazard ratio, 0.81).
Most significantly, death from cardiovascular causes, and death from any cause) did not differ significantly between the bempedoic acid and placebo.
Adverse effects
The overall incidences of adverse events, serious adverse events, and adverse events leading to discontinuation of the trial regimen did not differ meaningfully between the bempedoic acid group and the placebo group. In particular, myalgias were reported in 5.6% of the patients in the bempedoic acid group and in 6.8% of those in the placebo group.
Elevations in liver aminotransferase levels of more than three times the upper limit of the normal range occurred more frequently in the bempedoic acid group than in the placebo group, and the mean changes from baseline in the creatinine and uric acid levels were greater in the bempedoic acid group. The incidence of hyperuricemia was higher in the bempedoic acid group than in the placebo group (10.9% vs. 5.6%), as were the incidences of gout (3.1% vs. 2.1%) and cholelithiasis (2.2% vs. 1.2%).
Unlike statins, bempedoic acid, as compared with placebo, did not increase glycated hemoglobin levels or the incidence of new-onset diabetes. Six months of treatment with bempedoic acid resulted in a 21.6% reduction in the high-sensitivity CRP level relative to placebo.
In summary, bempedoic acid appears to modestly reduce LDL-C with a corresponding reduction in most cardiovascular outcomes although cardiovascular and non-cardiovascular deaths were not reduced in the trial. Musculo-skeletal adverse effects were not significantly different between the bempedoic acid and placebo groups although hyperuricaemia, gout and cholelithiasis were increased (although very small numbers) in the bempedoic acid group.
When bempedoic acid does come to Australia, we need to remember that statins remain the cornerstone of lipid lowering. Statins can reduce LDL-C by 50% more although this might necessitate high dose statins. For every mmol/L LDL-C reduction, there is a 20% reduction in adverse cardiovascular outcomes. It doesn’t matter how the reduction is achieved. PCSK9 inhibitors can also be used in select patients that qualify under the complicated PBS Authority criteria.
References:
- Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy — European Atherosclerosis Society Consensus Panel statement on assessment, aetiology and management. Eur Heart J 2015; 36: 1012-22.
- Bytyçi I, Penson PE, Mikhailidis DP, et al. Prevalence of statin intolerance: a meta-analysis. Eur Heart J 2022; 43: 3213-23.
- Hovingh GK, Gandra SR, McKendrick J, et al. Identification and management of patients with statin-associated symptoms in clinical practice: a clinician survey. Atherosclerosis 2016; 245: 111-7.
- Ray KK, Bays HE, Catapano AL, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med 2019; 380: 1022-32.
- Goldberg AC, Leiter LA, Stroes ESG, et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR Wisdom randomized clinical trial. JAMA 2019; 322: 1780-8.
- Ballantyne CM, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis 2018; 277: 195-203.
- Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc 2019; 8(7): e011662-112.
- Nissen SE, Lincoff AM, Brennan D, et al. Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. N Engl J Med. 2023 Mar 4. doi: 10.1056/NEJMoa2215024.
